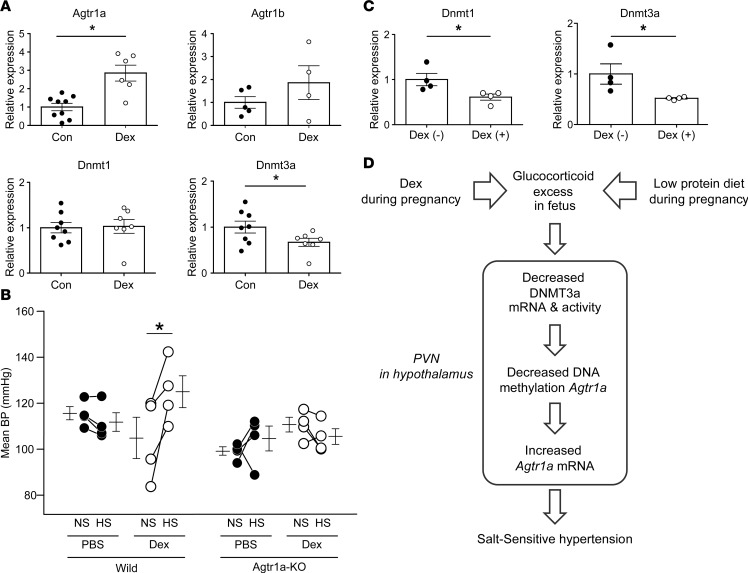
Figure 6. Offspring of pregnant C57BL6/J WT mice and pregnant Agtr1a-KO mice treated with dexamethasone (Dex).
(A) Real-time PCR of Agtr1a, Agtr1b, Dnmt1, and Dnmt3a mRNA in the paraventricular nucleus (PVN) of offspring of Dex-untreated (n = 6–9) and Dex-treated pregnant C57BL6/J mice (n = 4–7). Filled circles, Dex-untreated WT mice (Con); open circles, Dex-treated WT mice (Dex). (B) Mean BP by radiotelemetry before and after 1 week of HS treatment in offspring of pregnant WT mice (left) and pregnant Agtr1a-KO mice (right) treated with or without Dex. Filled circles, Dex-untreated WT mice and Agtr1a-KO mice; open circles, Dex-treated WT mice and Agtr1a-KO mice (WT, n = 4; Agtr1a-KO, n = 4). (C) Real-time PCR of Dnmt1 and Dnmt3a mRNA in Dex-untreated Agtr1a-KO mice (n = 4) and Dex-treated Agtr1a-KO mice (n = 4). Filled circles, Dex-untreated Agtr1a-KO mice; open circles, Dex-treated Agtr1a-KO mice. Throughout, data represent means ± SEM. In A and C, *P < 0.05 versus Dex-untreated WT mice or Dex-untreated Agtr1a-KO mice (t test); in B, *P < 0.05 versus NS-treated offspring of pregnant WT mice treated with Dex (2-way repeated ANOVA, Bonferroni post hoc text). (D) Model of prenatal programmed hypertension. A low-protein diet, as well as treatment of Dex, during pregnancy induces exposure to excessive glucocorticoid in the fetus, through the decreased 11β-HSD2 activity. Excessive glucocorticoid decreases Dnmt3a mRNA and activity, concomitant with DNA demethylation and Agtr1a upregulation in the PVN of hypothalamus of the offspring; the increased angiotensin signaling in the hypothalamus, in turn, develops salt-sensitive hypertension.