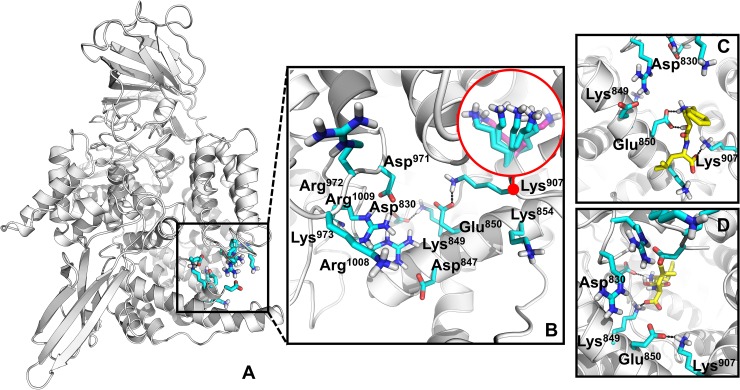
Fig 4. The C-terminal channel opening is made up of positively and negatively charged residues.
A: The overall structure of PfM1-AAP with the visualized charged residues of the channel entrance that coordinate the initial migration of the substrates and inhibitors. B: The zoomed view of the channel entrance. The flexibility of the Lys907 side chain is shown in the circle; conformations captured in sMD are in cyan and conformations observed in the crystal structures are in purple. C: Transient stable binding of Bestatin with Glu850 and Lys907. D: Transient stable binding of Bestatin with Lys849 and Asp830. The salt bridges are shown in a black-dotted line.