Abstract
Background:
Autosomal-dominant optic atrophy (ADOA) is one of the most common types of inherited optic atrophy. We identify OPA1 pathogenic variants and assess the clinical features of a cohort of Chinese ADOA patients
Materials and Methods:
Detailed clinical evaluations were performed and genomic DNA was extracted from peripheral blood for all the participants. Sanger sequencing was used to analyze all exons and exon/intron junctions of OPA1 for eight pedigrees. Target exome capture plus next-generation sequencing (NGS) were applied for one atypical family with photophobia. Reverse transcription polymerase chain reaction was carried out to further characterize the mRNA change of selected splicing alteration.
Results:
All 17 patients had impaired vision and optic-disk pallor; however, the clinical severity varied markedly. Two patients complicated with hearing loss. Six novel and two reported pathogenic variants in OPA1 (GenBank Accession No. NM_130837.2) were identified including four nonsynonymous variants(c.2400T > G, c.1468T > C, c.1567A > G and c.1466T > C), two splicing variants (c.2984-1_2986delGAGA and c.2983 + 5G > A), one small deletion (c.2960_2968delGCGTTCAAC), and one small insertion(c.3009_3010insA). RNA analysis revealed the splicing variant c.2984-1_2986delGAGA caused small deletion of mRNA (r.2983_2988del).
Conclusions:
ADOA patients presented variable clinical manifestations. Novel OPA1 pathogenic variants are the main genetic defect for Chinese ADOA cases. NGS may be a useful molecular testing tool for atypical ADOA.
Keywords: ADOA, hearing loss, NGS, OPA1
Introduction
Autosomal-dominant optic atrophy (ADOA) or Kjer’s disease (OMIM 165500) is one of the most common types of genetically inherited optic atrophy, with an estimated prevalence between 1:12000 and 1:50000 worldwide (1,2). Typical ADOA patients manifest painless, symmetric, and insidious vision loss from childhood. Visual acuity may progressively decline but mostly remains stable around 0.1 (can range from hand motion to 1.0) (3). The optic disks tend to show temporal pallor or diffuse pallor. Varying degrees of color vision defects and predominantly central or paracentral scotoma can be detected. About 20–30% of ADOA patients may present other manifestations besides optic atrophy, such as sensorineural deafness, progressive external ophthalmoplegia, myopathy, and ataxia (4). If patients manifest one or more additional features, they are referred to as ADOA plus.
So far, eight genes or loci related to dominant optic atrophy have been identified (5–12). OPA1 gene variants contribute to 57–89% of ADOA (13–15). The OPA1 gene is located on 3q28, which consists of 30 coding exons (16). Alternative splicing generates at least seven isoforms. It encodes a dynamin-related GTPase family protein located in the inner membrane of mitochondria. The OPA1 protein is composed of four functional domains: basic domain (exon1–3), GTPase domain (exon10–17), dynamin central domain (exon18–26), and putative GTPase effector domain (GED) (exon 29–30). The OPA1 protein has a key role in maintaining the balance between mitochondrial fissure and fusion, cristae remodeling, and maintaining normal respiratory chain potential (17–19). It also participates in the mitochondrial anti-apoptosis process (20,21). There are more than 340 reported OPA1 pathogenic variants (eOPA1 database: http://mitodyn.org/home.php? select_db=OPA1), most of which are splicing variants, mis-sense/nonsense variants, and deletions (5).
The data of large cohort studies of Chinese families with hereditary optic neuropathy showed the proportion of OPA1-related DOA is much lower than that of Leber hereditary optic neuropathy (LHON, OMIM 535000) (7.9% versus45.2%) (22,23), which is different from Caucasian groups(30.1% for OPA1-related DOA and 13.3% for LHON) (15). The authors suggested that lack of awareness, especially mild or atypical phenotypes of DOA, in Chinese population might explain the low detection rate. In this study, we described the clinical features and molecular characterizations of eight Chinese families with ADOA.
Methods
Clinical evaluation and DNA preparation
Eight Chinese families of Han ethnicity exhibiting ADOA were identified at the Ophthalmic Genetic Clinic at Peking Union Medical College Hospital (PUMCH). Detailed medical and family history was taken. The patients underwent ophthalmologic evaluations, including best-corrected visual acuity (BCVA) according to decimal Snellen E chart, color vision, intraocular pressure, slit-lamp biomicroscopy, dilated ophthalmoscopy, optical coherence tomography (OCT) (Heidelberg HRT II, Heidelberg, Germany), and visual-field testing (Octopus, Interzeag, Schlieren, Switzerland). Pattern-ERG (pERG) and visual evoked potential (VEP) (RetiPort ERG system, Roland Consult, Wiesbaden, Germany) were performed and complied with the standard published by the International Society for Clinical Electrophysiology of Vision (www.iscev.org). Audiogram testing was performed for the patients with suspected hearing loss. Genomic DNA was isolated from peripheral blood with QIAamp DNA Blood Midi Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Informed consent was obtained from all participants. This study was approved by the Institutional Review Board of PUMCH and adhered to the tenets of the Declaration of Helsinki and the Guidance on Sample Collection of Human Genetic Diseases by the Ministry of Public Health of China.
Sequence screening of OPA1 gene
Polymerase chain reactions (PCR) were designed to amplify OPA1 exons and splice-site sequences. Primers were synthesized according to sequences published previously (22). The final volume of 50 μl contained 40 ng genomic DNA, 10 pmol of each primer, and 25 μl 2 × Taq PCR Master Mix (Transgene Technologies, Beijing, China). The amplification was performed under the following conditions: 95°C for 5 min, followed by 33 cycles at 95°C for 30 s, 60°C for 30 s, 72°C for 45 s, with a final extension at 72°C for 7 min. After purification, amplicons were sequenced using forward and reverse primers on an ABI 3730 Genetic Analyzer (ABI, Foster City, CA). The sequences were assembled and analyzed using Lasergene SeqMan software (DNASTAR, Madison, WI). The results were compared with the OPA1 transcript (GenBank Accession No. NM_130837.2). All available family members were Sanger sequenced in order to perform segregation tests.
Capture sequencing, data analysis, and Sanger verification
Approximately 1 μg of the genomic DNA sample of the proband was sheared into fragments of 200–500 bp in length. The sheared fragments were blunt-end repaired and a single-adenine base was added to the 3′ends using Klenow exonuclease. Illumina adapters were ligated to the repaired ends and DNA fragments were PCR amplified for eight cycles after ligation. In each capture reaction, 50 pre-capture DNA libraries were pooled together. The targeted DNA was captured by a customized disease gene panel to screen for variants in known retinal disease-causing genes. The detailed information of the capture panel was described previously (24). Captured libraries were sequenced on Illumina HiSeq 2000 (Illumina, San Diego, CA, USA) as 100 bp paired-end reads according to the manufacturer’s protocol.
Paired-end sequencing reads were obtained for each sample. Reads were mapped to human reference genome hg19 using Burrows-Wheeler Aligner (25). Base quality recalibration, local realignment, and variant calling were performed as previously described (24). We obtained the variant frequencies from a series of public and internal control databases as well as the Exome Aggregation Consortium database. Variants with a frequency >.5% were filtered out. After frequency-based filtering, we filtered out synonymous variants, identified known retinal disease-causing variants and predicted the pathogenicity of variants using sorting intolerant from tolerant (SIFT) (26), Polyphen2 (27), likelihood ratio test (LRT) (28), MutationTaster (29), MutationAssessor (30) as previously described (24,31).
The causative variants were validated and co-segregation was confirmed by Sanger sequencing.
RNA analysis
Total RNA was extracted from periphery blood using QIAamp RNA Blood Midi Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. RNA was reverse transcribed to single-stranded cDNA using GoScript™ Reverse Transcription System (Promega Corporation, Madison, WI). PCR primers were designed to amplify the segments encompassing splicing variant c.2984-1_2986delGAGA (F1: TTGCTGAAGATGGTGAGAAGAA, R1: AGGCTGGACAAAAGACGTTG) with Primer 3 (http://frodo.wi.mit.edu/). RT-PCR products were directly sequenced on an ABI 3730 Genetic Analyzer.
Results
Clinical evaluation
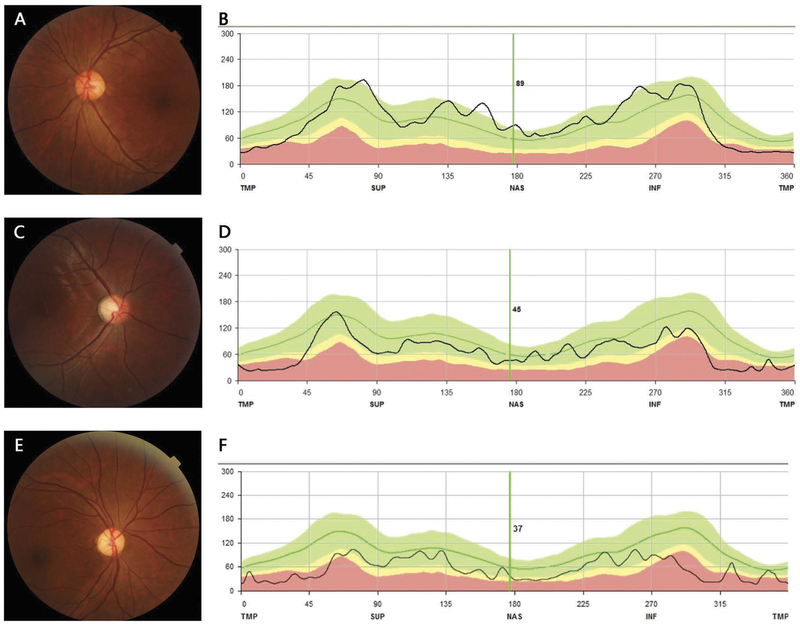
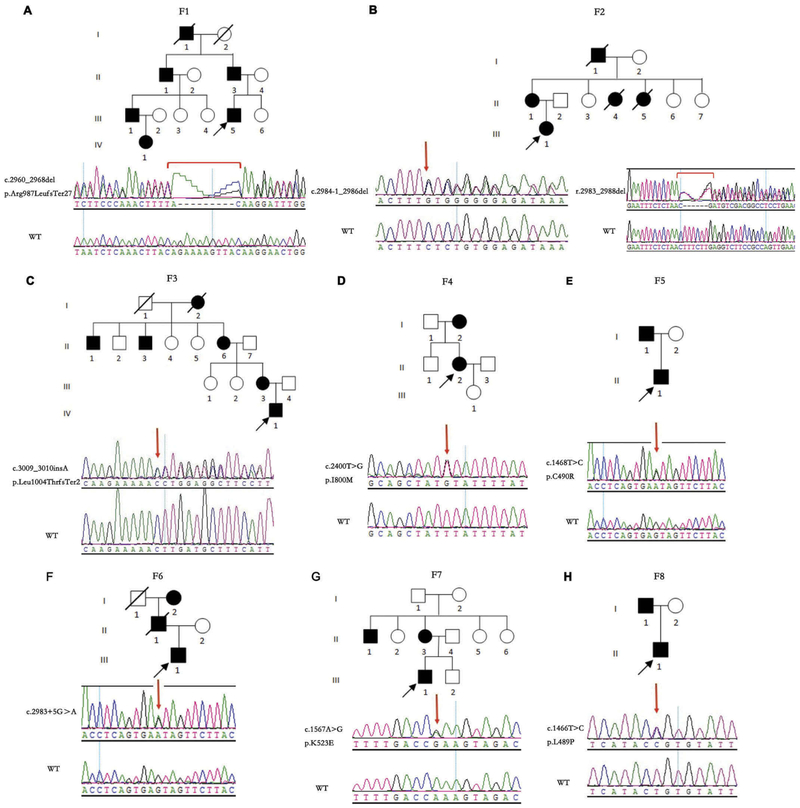
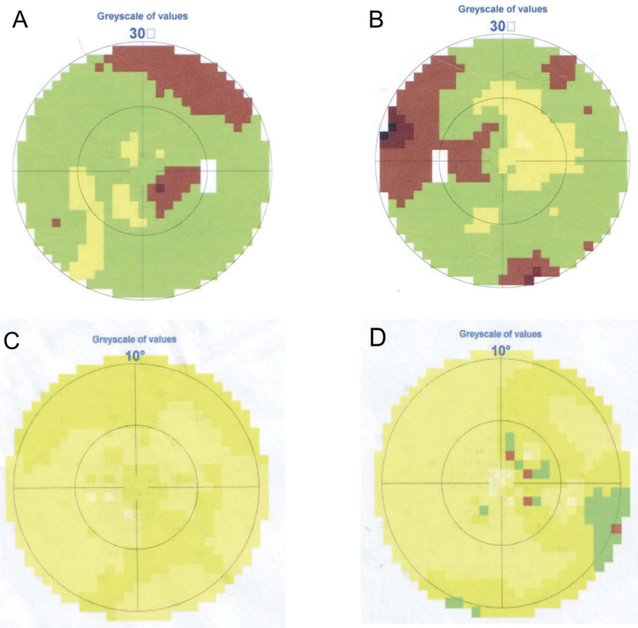
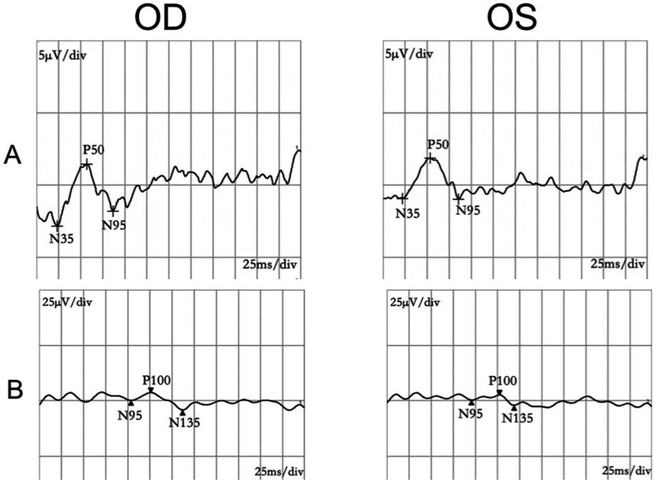
Eight pedigrees exhibited autosomal-dominant transmission (Figure 1). Patients’ ages ranged from 1 to 61 years old. Though the 1-year-old patient F1-VI:1 couldn’t cooperate with visual-acuity examination, she failed to follow large objects precisely. F7-II:3 had BCVA of 0.25 OU with no chief complaint of declined visual function. She came to visit our clinic because of her son with optic atrophy. All the other 15 patients experienced progressively declined poor vision at an early age. The BCVA ranged from light perception to 0.4. Color vision could be normal or varying degrees of dyschromatopsia. Fundus examination revealed normal appearing, temporal pallor or total pallor of the optic nerve head (Figure 2(a,c,e)). OCT showed diffuse or temporal thinning of retinal nerve fiber layer (RNFL) (Figure 2(b,d,f)). All visual-field testing showed central or paracentral scotoma except for F7-II:3, who presented with a normal visual field in the right eye (Figure 3). Abnormal P-ERG and VEP were recorded with decreased amplitude of N95 wave and prolonged P100 peak time and reduced amplitude (Figure 4). A normal P-ERG OU and a normal VEP OS were recorded in F7-II:3. Four patients had nystagmus. The proband from F8 complained of obvious photophobia besides vision impairment. Hearing loss was present in two patients from independent families (F3-II:6, F5-I:1). Audiogram testing showed F3-II:6 had sensorineural deafness. The detailed clinical findings of affected members of each family were summarized in Table 1.
Figure 1.
Pedigrees and sequencing results of the 8 ADOA families. (A) F1-II:3,F1-III:1, F1-III:5, and F1-VI:1 carried the same heterozygous c.2960_2968del GCGTTCAAC (p.Arg987LeufsTer27) variant. (B) F2-II:1 and F2-III:1 carried the same heterozygous c.2984-1_2986delGAGA variant. An in-frame small deletion was identified in F2-III:1ʹ s mRNA (r.2983_2988del). (C) F3-II:6, F3-III:3, and F3-IV:1 carried the same heterozygous c.3009_3010insA (p.Leu1004ThrfsTer2) variant. (D) F4-II:2 carried the heterozygous c.2400T > G (p.I800M) variant. (E) F5-I:1 and F5-II:1 carried the same heterozygous c.1468T > C (p.C490R) variant. (F) F6-III:1 carried the heterozygous c.2983 + 5G>A variant. (G) F7-II:3 and F7-III:1 carried the same heterozygous c.1567A > G (p.K523E) variant. (H) F8-I:1 and F8-II:1 carried the same heterozygous c.1466T > C (p.L489P) variant. WT refers to wildtype; squares indicate men; circles indicate women; black indicates affected subjects; symbols with a slash indicate deceased subjects.
Figure 2.
Fundus photographs and OCT images of representative patients. F7-II:3, 37-year-old female, mild temporal pallor of ONH (A), slight thinning of temporal RNFL (B). F7-III:1, 12-year-old male, typical temporal pallor of ONH (C), temporal thinning of RNFL (D). F2-III:1, 34-year-old female, diffuse pallor of ONH (E), diffuse thinning of RNFL (F).
Figure 3.
Visual fields of F2-III:1 (A, B) and F7-II:3 (C, D). (A) central scotoma, OD. (B) central and paracentral scotoma, OS. (C) normal central visual field, OD. (D) central scotoma, OS.
Figure 4.
P-ERG(A) and VEP(B) of F4-II:2. Declined amplitude of N95 wave and prolonged latent P100 peak time and reduced amplitude, both eyes.
Table 1.
Summary of clinical findings of the patients.
| Patient | sex/age | BCVA (OD/OS) |
CV defect |
ONH | RNFL (OD/OS) |
VF | P-ERG | VEP | other |
|---|---|---|---|---|---|---|---|---|---|
| Fl-III:5 | M/24 | 0.04/0.04 | Y | DP | DT | NA | Amp-N95↓ | Lat-P100TAmp-P100↓ | nystagmus,strabismus |
| F1-II:3 | M/45 | 0.01/0.01 | Y | DP | DT | NA | NA | NA | nystagmus,strabismus |
| Fl-III:1 | M/39 | 0.02/0.02 | Y | DP | NA | NA | NA | NA | nystagmus,strabismus |
| F1-VI:1 | F/1 | NA | NA | DP | NA | NA | NA | NA | |
| F2-III:1 | F/34 | 0.08/0.08 | Y | DP | TT | CS,PS | Amp-N95↓ | Lat-P100↑Amp-P100↓ | |
| F2-II:1 | F/61 | 0.12/0.12 | Y | DP | TT | NA | Amp-N95↓ | Lat-P100↑Amp-P100↓ | |
| F3-IV:1 | M/5 | 0.1/0.2 | Y | TP | NA | NA | Amp-N95↓ | Lat-P100↑Amp-P100↓ | |
| F3-III:3 | F/26 | 0.1/0.12 | Y | TP | TT | CS | Amp-N95↓ | Lat-P100↑Amp-P100↓ | |
| F3-II:6 | F/58 | 0.15/0.2 | Y | TP | TT | NA | Amp-N95↓ | Lat-P100↑Amp-P100↓ | hearing loss |
| F4-II:2 | F/45 | 0.4/0.4 | Y | TP | TT | CS | Amp-N95↓ | Lat-P100↑Amp-P100↓ | |
| F5-II:1 | M/4 | 0.05/0.05 | NA | DP | NA | NA | NA | NA | nystagmus |
| F5-I:1 | M/43 | LP/LP | NA | DP | NA | NA | NA | NA | hearing loss |
| F6-III:1 | M/13 | 0.3/0.3 | N | TP | TT | CS | NA | NA | |
| F7-III:1 | M/12 | 0.15/0.25 | NA | N | TT | CS | Amp-N95↓ | Lat-P100↑Amp-P100↓ | |
| F7-II:3 | F/36 | 0.25/0.25 | NA | TP | TT | CS,PS | N | Lat-P100↑Amp-P100↓(OD),N(OS) | |
| F8-II:1 | M/6 | 0.05/0.05 | Y | TP | DT | CS | Amp-N95↓ | Lat-P100↑Amp-P100↓ | photophobia |
| F8-I:1 | M/39 | 0.2/0.3 | Y | TP | DT | NA | Amp-N95↓ | Lat-P100↑Amp-P100↓ |
BCVA, best corrected visual acuity. CV, color vision. ONH, optic nerve head. RNFL, retinal nerve fiber layer. VF, visual field. P-ERG, pattern electroretinogram. VEP, visual evoked potential. DP, diffuse pallor. TP, temporal pallor. DT, diffuse thinning. TT, temporal thinning. CS, central scotoma. PS, paracentral scotoma. Amp-N95, amplitude of N95 wave. Lat-P100, latent P100 peak time. Amp-P100, amplitude of P100 peak time. Y, yes. N, normal. NA, nonavailable.
Genetic analysis
Direct screening of the OPA1 gene (GenBank Accession No. NM_130837.2) together with next-generation sequencing identified four nonsynonymous pathogenic variants (c.2400T > G, c.1468T > C, c.1567A > G and c.1466T > C), two splicing variants (c.2984-1_2986delGAGA and c.2983 + 5G > A), one small deletion (c.2960_2968delGCGTTCAAC), and one small insertion (c.3009_3010insA). These variants co-segregated with the disease in the pedigrees (Figure 1). The four novel nonsynonymous variants were predicted as pathogenic by SIFT, Polyphen2, and Mutation Taster. For the splicing variant c.2984-1_2986del, reverse transcription polymerase chain reaction displayed an in-frame small deletion of the patient’s mRNA (r.2983_2988del) (Figure 1(b)). The results of genetic analysis were summarized in Table 2.
Table 2.
Summary of the genetic analysis of the eight ADOA families.
| Family | Exon | DNA mutation |
Protein/mRNA change | Mutation type |
SIFT | P0LYPHEN2 | MUTATION TASTER | Novel |
|---|---|---|---|---|---|---|---|---|
| F1 | 29 | c.2960_2968del GCGTTCAAC | p.Arg987LeufsTer27 | small deletion | — | — | DC | Y |
| F2 | 30 | c.2984-1_2986delGAGA | r.2983_2988del | splicing | — | — | DC | N |
| F3 | 30 | c.3009_3010insA | Pleu1004ThrfsTer2 | small insertion | — | — | DC | Y |
| F4 | 24 | c.2400T > G | p.l800M | missense | 0.01 (D) | 0.987(PD) | DC | Y |
| F5 | 15 | c.1468T > C | p.C490R | missense | 0.0(D) | 1(PD) | DC | Y |
| F6 | 29 | c.2983 + 5G>A | — | splicing | — | — | DC | N |
| F7 | 16 | c.1567A > G | P.K523E | missense | (D) | 0.948(PD) | DC | Y |
| F8 | 15 | c.1466T > C | p.L489P | missense | 0.0(D) | 1(PD) | DC | Y |
D, damaging. PD, probably damaging. DC, disease causing. Y, yes. N, no. All the variants are described according to OPA1 transcript variant 8 (NM_130837.2)
Discussion
Autosomal-dominant optic atrophy is an inherited eye disease causing serious impact on visual function and quality of life. Understanding the underlying genetic defect is the first step for a better intervention. In this study, 17 Chinese subjects from eight unrelated families, all presented with poor vision since childhood with 41% legally blind. Genetic analysis revealed a total of eight pathogenic variants in the OPA1 gene. Nonsynonymous variant (50%) was the most common type of sequence variation followed by splicing (25%), which is similarity to the human gene mutation database (HGMD) (http://www.hgmd.cf.ac.uk/ac/index.php). The OPA1 studies on European populations showed that gross deletions/insertions accounted for about one fifth of the negative cases (32,33). Though the proportion of gross genomic aberration in Chinese ADOA patients was not fully evaluated, it was estimated to be higher than 12% (23). There might also be variants that were undetectable by multiplex ligation-dependent probe amplification (MLPA). The pathogenic variants identified in our study didn’t include c.2708_2711del, which was a hot-spot pathogenic variant in both Chinese and European ADOA groups, accounting for 10.8%~27.2% of the total variants (13,22,23,34). The stop-gain and frameshift variants that lead to the premature translation termination of OPA1 were predicted to undergo nonsense-mediated mRNA decay (35), which suggested the pathogenesis of haploinsufficiency. Most missense pathogenic variants have been reported in the OPA1 gene are clustered in the GTPase domain, which may be caused by dominant negative effect.
The severity of the phenotypes varied greatly among and between the eight Chinese ADOA families, consistent to other studies (36–38). BCVA of the ADOA patients in our study ranged from light perception to 0.4. It could range from light perception to 1.0 according to previous reports (39). Color vision was abnormal in all our patients except for F6-III:1. Almost all ADOA patients manifest color vision defect, mostly generalized dyschromatopsia (40). The optic-disk pallor in DOA falls into two main categories including diffuse pallor and temporal pallor (41). Eight of 17 patients in our cohort showed temporal optic-disk pallor. Seven of these eight patients had the BCVA greater than 0.1, whereas the BCVA of seven of the eight patients with diffuse optic-disk pallor were less than 0.1. This suggests that temporal optic-disk pallor may be correlated with better visual function than diffuse optic-disk pallor. OCT is an effective and convenient tool to access the structure of retina. All our patients revealed varying degrees of thin RNFL by OCT scan, conforming to different severities of optic atrophy. For those who present mild optic atrophy, visual field and electrophysiology tests (including Pattern ERG and VEP) are recommended in detecting the damaged function of RGC. F7-II:3 was an asymptomatic ADOA patient with no major complain of declined visual acuity. She presented with a normal appearing optic nerve head and slight temporal thinning of RNFL. Asymptomatic patients are not rare in ADOA (37,42). It has been reported that some DOA patients may display only subtle optic-disk atrophy or even a normal-looking optic disk (43). Patients like F7-II:3 may be easily ignored. Thus, careful and comprehensive clinical examinations as well as molecular analysis of suspected patients are needed.
Similar to the mitochondrial DNA-related disease LHON, ADOA patients may also present some other neurologic abnormalities except for optic atrophy, such as sensorineural deafness, ophthalmoplegia, myopathy, and ataxia (4,44). Among which, sensorineural deafness is the most common (60%) extra-ocular manifestation (44). Hearing loss was recorded in our ADOA cohort (2/17). The 58-year-old female patient (F3-II:6) and the 43-year-old male patient (F5-I:1) from different families complicated with this feature. Intraand inter-family variations in this phenotype were obvious. The audiogram of F3-II:6 showed sensorineural deafness in both ears, whereas the audiograms of her affected daughter and grandson were normal. We identified a frameshift variant in F3 (c.3009_3010insA) and a missense variant in F5(c.1468T > C). More than 10 variants identified were linked with ADOA and hearing loss phenotype, of specially, p. R445H in exon14 (44–47). However, both inter- and intra-familiar variations were obvious. For instance, some families carrying p.R445H did not present with hearing loss (48,49). Except for hearing loss, none of our patients showed any other extra-ocular features. Notably, even though F2 and one family reported by Ranieri et al. (50) shared the same pathogenic variant of c.2984-1_2986delGAGA, the latter one had some neurological abnormalities including bilateral ptosis, ophthalmoparesis, and a decreased/absent Achilles tendon reflex. Additional genetic or environmental modifier factors may contribute to the phenotypic varieties in ADOA.
For the typical cases, DOA patients manifest painless, symmetric, and insidious vision loss from childhood. Atypical DOA may be misdiagnosed in some condition. The proband in F8 was a 5-year-old boy. He complained of obvious photophobia and progressively declined vision and was diagnosed as cone dystrophy before he was referred to our clinic. We performed next-generation sequencing using the customized retinal disease gene panel and identified a novel missense variant (c.1466T >C) in the OPA1 gene. He was finally re-diagnosed as ADOA. Photophobia is not a common feature of ADOA, which has never been reported so far. Next-generation sequencing is an effective and efficient way to make the right diagnosis especially when the clinical manifestations are atypical.
In summary, this study identified six novel and two reported pathogenic variants of the OPA1 gene in Chinese ADOA patients. All the 17 patients in eight unrelated families had poor vision and optic-disk pallor; however, the clinical manifestations varied in severity. As the phenotypes of ADOA can be very mild, a routine examination of the suspected relatives should not be ignored. Hearing loss is not rare in ADOA, so much so the consultation of otology was often required. For those who have atypical phenotypes, next-generation sequencing can be used to help make the right diagnosis. A comprehensive understanding of the clinical and genetic spectrum of ADOA would certainly facilitate the diagnosis as well as development of therapeutic strategies.
Acknowledgments
We would like to thank the patients who participated in the study.
Funding
This work was supported by the National Natural Science Foundation of China (81470669), Beijing Natural Science Foundation (7152116), the Ministry of Science and Technology of the People’s Republic of China (2010DFB33430), the Foundation Fighting Blindness USA (CD-CL-0214-0631-PUMCH), and CAMS Innovation Fund for Medical Sciences (CIFMS 2016-12M-1-002).
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- 1.Fraser JA, Biousse V, Newman NJ. The neuro-ophthalmology of mitochondrial disease. Surv Ophthalmol. 2010;55(4):299–334. doi:10.1016/j.survophthal.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kjer B, Eiberg H, Kjer P, Rosenberg T. Dominant optic atrophy mapped to chromosome 3q region. II. Clinical and epidemiological aspects. Acta Ophthalmol Scand. 1996;74(1):3–7. doi:10.1111/j.1600-0420.1996.tb00672.x. [DOI] [PubMed] [Google Scholar]
- 3.Yu-Wai-Man P, Griffiths PG, Burke A, Sellar PW, Clarke MP, Gnanaraj L, Ah-Kine D, Hudson G, Czermin B, Taylor RW, et al. The prevalence and natural history of dominant optic atrophy due to OPA1 mutations. Ophthalmology. 2010;117(8):1538–1546, 1546.e1531. doi:10.1016/j.ophtha.2009.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skidd PM, Lessell S, Cestari DM. Autosomal dominant hereditary optic neuropathy (ADOA): a review of the genetics and clinical manifestations of ADOA and ADOA+. Semin Ophthalmol. 2013;28(5–6):422–26. doi:10.3109/08820538.2013.825296. [DOI] [PubMed] [Google Scholar]
- 5.Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26(2):211–15. doi:10.1038/79944. [DOI] [PubMed] [Google Scholar]
- 6.Kerrison JB, Arnould VJ, Ferraz Sallum JM, Vagefi MR, Barmada MM, Li Y, Zhu D, Maumenee IH. Genetic heterogeneity of dominant optic atrophy, Kjer type: identification of a second locus on chromosome 18q12.2-12.3. Arch Ophthalmol. 1999;117(6):805–10. doi:10.1001/archopht.117.6.805. [DOI] [PubMed] [Google Scholar]
- 7.Katz BJ, Zhao Y, Warner JE, Tong Z, Yang Z, Zhang K. A family with X-linked optic atrophy linked to the OPA2 locus Xp11.4-Xp11.2. Am J Med Genet A. 2006;140(20):2207–11. doi:10.1002/ajmg.a.31455. [DOI] [PubMed] [Google Scholar]
- 8.Anikster Y, Kleta R, Shaag A, Gahl WA, Type EO. III 3-methylgluta-conic aciduria (optic atrophy plus syndrome, or Costeff optic atrophy syndrome): identification of the OPA3 gene and its founder mutation in Iraqi Jews. Am J Hum Genet. 2001;69(6):1218–24. doi:10.1086/324651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carelli V, Schimpf S, Fuhrmann N, Valentino ML, Zanna C, Iommarini L, Papke M, Schaich S, Tippmann S, Baumann B, et al. A clinically complex form of dominant optic atrophy (OPA8) maps on chromosome 16. Hum Mol Genet. 2011;20(10):1893–905. doi:10.1093/hmg/ddr071. [DOI] [PubMed] [Google Scholar]
- 10.Hanein S, Perrault I, Roche O, Gerber S, Khadom N, Rio M, Boddaert N, Jean-Pierre M, Brahimi N, Serre V, et al. TMEM126A, encoding a mitochondrial protein, is mutated in autosomal-recessive nonsyndromic optic atrophy. Am J Hum Genet. 2009;84(4):493–98. doi:10.1016/j.ajhg.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbet F, Hakiki S, Orssaud C, Gerber S, Perrault I, Hanein S, Ducroq D, Dufier JL, Munnich A, Kaplan J, et al. A third locus for dominant optic atrophy on chromosome 22q. J Med Genet. 2005;42(1):e1. doi:10.1136/jmg.2004.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbet F, Gerber S, Hakiki S, Perrault I, Hanein S, Ducroq D, Tanguy G, Dufier J-L, Munnich A, Rozet J-M, et al. A first locus for isolated autosomal recessive optic atrophy (ROA1) maps to chromosome 8q. Eur J Hum Genet. 2003;11(12):966–71. doi:10.1038/sj.ejhg.5201070. [DOI] [PubMed] [Google Scholar]
- 13.Delettre C, Griffoin J-M, Kaplan J, Dollfus H, Lorenz B, Faivre L, Lenaers G, Belenguer P, Hamel CP. Mutation spectrum and splicing variants in the OPA1 gene. Hum Genet. 2001;109(6):584–91. doi:10.1007/s00439-001-0633-y. [DOI] [PubMed] [Google Scholar]
- 14.Toomes C, Marchbank NJ, Mackey DA, Craig JE, Newbury-Ecob RA, Bennett CP, Vize CJ, Desai SP, Black GC, Patel N, et al. Spectrum, frequency and penetrance of OPA1 mutations in dominant optic atrophy. Hum Mol Genet. 2001;10(13):1369–78. doi:10.1093/hmg/10.13.1369. [DOI] [PubMed] [Google Scholar]
- 15.Ferre M, Bonneau D, Milea D, Chevrollier A, Verny C, Dollfus H, Ayuso C, Defoort S, Vignal C, Zanlonghi X, et al. Molecular screening of 980 cases of suspected hereditary optic neuropathy with a report on 77 novel OPA1 mutations. Hum Mutat. 2009;30(7):E692–705. doi:10.1002/humu.v30:7. [DOI] [PubMed] [Google Scholar]
- 16.Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, Pelloquin L, Grosgeorge J, Turc-Carel C, Perret E, et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26(2):207–10. doi:10.1038/79936. [DOI] [PubMed] [Google Scholar]
- 17.Ranieri M, Brajkovic S, Riboldi G, Ronchi D, Rizzo F, Bresolin N, Corti S, Comi GP. Mitochondrial fusion proteins and human diseases. Neurol Res Int. 2013;2013:293893. doi:10.1155/2013/293893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liesa M, Palacin M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89(3):799–845. doi:10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 19.Frezza C, Cipolat S. Martins de Brito O, et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126(1):177–89. doi:10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 20.Landes T, Leroy I, Bertholet A, Diot A, Khosrobakhsh F, Daloyau M, Davezac N, Miquel M-C, Courilleau D, Guillou E. OPA1 (dys) functions. Semin Cell Dev Biol. 2010;21(6):593–98. doi:10.1016/j.semcdb.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, Lenaers G. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem. 2003;278(10):7743–46. doi:10.1074/jbc.C200677200. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Jia X, Wang P, Xiao X, Li S, Guo X, Zhang Q. Mutation survey of the optic atrophy 1 gene in 193 Chinese families with suspected hereditary optic neuropathy. Mol Vis. 2013;19:292–302. [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Xu K, Zhang X, Jiang F, Liu L, Dong B, Ren Y, Li Y. Mutation screening of mitochondrial DNA as well as OPA1 and OPA3 in a Chinese cohort with suspected hereditary optic atrophy. Invest Ophthalmol & Vis Sci. 2014;55(10):6987–95. doi:10.1167/iovs.14-14953. [DOI] [PubMed] [Google Scholar]
- 24.Wang F, Wang H, Tuan HF, Nguyen DH, Sun V, Keser V, Bowne SJ, Sullivan LS, Luo H, Zhao L, et al. Next generation sequencing-based molecular diagnosis of retinitis pigmentosa: identification of a novel genotype-phenotype correlation and clinical refinements. Hum Genet. 2014;133(3):331–45. doi:10.1007/s00439-013-1381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics (Oxford, England). 2009;25(14):1754–60. doi:10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31(13):3812–14. doi:10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–49. doi:10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chun S, Fay JC. Identification of deleterious mutations within three human genomes. Genome Res. 2009;19(9):1553–61. doi:10.1101/gr.092619.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwarz JM, Rodelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010;7(8):575–76. doi:10.1038/nmeth0810-575. [DOI] [PubMed] [Google Scholar]
- 30.Reva B, Antipin Y, Sander C. Predicting the functional impact of protein mutations: application to cancer genomics. Nucleic Acids Res. 2011;39(17):e118. doi:10.1093/nar/gkr407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu M, Gelowani V, Eblimit A, Wang F, Young MP, Sawyer BL, Zhao L, Jenkins G, Creel DJ, Wang K, et al. ATF6 is mutated in early onset photoreceptor degeneration with macular involvement. Invest Ophthalmol & Vis Sci. 2015;56(6):3889–95. doi:10.1167/iovs.15-16778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuhrmann N, Alavi MV, Bitoun P, Woernle S, Auburger G, Leo-Kottler B, Yu-Wai-Man P, Chinnery P, Wissinger B. Genomic rearrangements in OPA1 are frequent in patients with autosomal dominant optic atrophy. J Med Genet. 2009;46(2):136–44. doi:10.1136/jmg.2008.062570. [DOI] [PubMed] [Google Scholar]
- 33.Almind GJ, Gronskov K, Milea D, Larsen M, Brondum-Nielsen K, Ek J. Genomic deletions in OPA1 in Danish patients with auto-somal dominant optic atrophy. BMC Med Genet. 2011;12:49. doi:10.1186/1471-2350-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thiselton DL, Alexander C, Taanman JW, Brooks S, Rosenberg T, Eiberg H, Andreasson S, Van Regemorter N, Munier FL, Moore AT, et al. A comprehensive survey of mutations in the OPA1 gene in patients with autosomal dominant optic atrophy. Invest Ophthalmol & Vis Sci. 2002;43(6):1715–24. [PubMed] [Google Scholar]
- 35.Schimpf S, Fuhrmann N, Schaich S, Wissinger B. Comprehensive cDNA study and quantitative transcript analysis of mutant OPA1 transcripts containing premature termination codons. Hum Mutat. 2008;29(1):106–12. doi:10.1002/humu.v29:1. [DOI] [PubMed] [Google Scholar]
- 36.Kamakari S, Koutsodontis G, Tsilimbaris M, Fitsios A, ChrousosG. First report of OPA1 screening in Greek patients with auto-somal dominant optic atrophy and identification of a previously undescribed OPA1 mutation. Mol Vis. 2014;20:691–703. [PMC free article] [PubMed] [Google Scholar]
- 37.Hoyt CS. Autosomal dominant optic atrophy. A spectrum of disability. Ophthalmology. 1980;87(3):245–51. doi:10.1016/S0161-6420(80)35247-0. [DOI] [PubMed] [Google Scholar]
- 38.Hamahata T, Fujimaki T, Fujiki K, Miyazaki A, Mizota A, Murakami A. OPA1 mutations in Japanese patients suspected to have autosomal dominant optic atrophy. Jpn J Ophthalmol. 2012;56(1):91–97. doi:10.1007/s10384-011-0096-1. [DOI] [PubMed] [Google Scholar]
- 39.Votruba M, Fitzke FW, Holder GE, Carter A, Bhattacharya SS, Moore AT. Clinical features in affected individuals from 21 pedigrees with dominant optic atrophy. Arch Ophthalmol. 1998;116(3):351–58. doi:10.1001/archopht.116.3.351. [DOI] [PubMed] [Google Scholar]
- 40.Yu-Wai-Man P, Griffiths PG, Hudson G, Chinnery PF. Inherited mitochondrial optic neuropathies. J Med Genet. 2009;46(3):145–58. doi:10.1136/jmg.2007.054270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Votruba M, Thiselton D, Bhattacharya SS. Optic disc morphology of patients with OPA1 autosomal dominant optic atrophy. Br J Ophthalmol. 2003;87(1):48–53. doi:10.1136/bjo.87.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohn AC, Toomes C, Hewitt AW, Kearns LS, Inglehearn CF, Craig JE, Mackey DA. The natural history of OPA1-related auto-somal dominant optic atrophy. Br J Ophthalmol. 2008;92(10):1333–36. doi:10.1136/bjo.2007.134726. [DOI] [PubMed] [Google Scholar]
- 43.Cohn AC, Toomes C, Potter C, Towns KV, Hewitt AW, Inglehearn CF, Craig JE, Mackey DA. Autosomal dominant optic atrophy: penetrance and expressivity in patients with OPA1 mutations. Am J Ophthalmol. 2007;143(4):656–62. doi:10.1016/j.ajo.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 44.Yu-Wai-Man P, Griffiths PG, Gorman GS, Lourenco CM, Wright AF, Auer-Grumbach M, Toscano A, Musumeci O, Valentino ML, Caporali L, et al. Multi-system neurological disease is common in patients with OPA1 mutations. Brain. 2010;133(3):771–86. doi:10.1093/brain/awq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liskova P, Ulmanova O, Tesina P, Melsova H, Diblik P, Hansikova H, Tesarova M, Votruba M. Novel OPA1 missense mutation in a family with optic atrophy and severe widespread neurological disorder. Acta Ophthalmol. 2013;91(3):e225–231. doi:10.1111/aos.2013.91.issue-3. [DOI] [PubMed] [Google Scholar]
- 46.Chen S, Zhang Y, Wang Y, Li W, Huang S, Chu X, Wang L, Zhang M, Liu Z. A novel OPA1 mutation responsible for auto-somal dominant optic atrophy with high frequency hearing loss in a Chinese family. Am J Ophthalmol. 2007;143(1):186–88. doi:10.1016/j.ajo.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 47.Namba K, Mutai H, Takiguchi Y, Yagi H, Okuyama T, Oba S, Yamagishi R, Kaneko H, Shintani T, Kaga K, et al. Molecular impairment mechanisms of novel OPA1 mutations predicted by molecular modeling in patients with autosomal dominant optic atrophy and auditory neuropathy spectrum disorder. Otol Neurotol. 2016;37(4):394–402. [DOI] [PubMed] [Google Scholar]
- 48.Shimizu S, Mori N, Kishi M, Sugata H, Tsuda A, Kubota N. A novel mutation in the OPA1 gene in a Japanese patient with optic atrophy. Am J Ophthalmol. 2003;135(2):256–57. doi:10.1016/S0002-9394(02)01929-3. [DOI] [PubMed] [Google Scholar]
- 49.Payne M, Yang Z, Katz BJ, Warner JEA, Weight CJ, Zhao Y, Pearson ED, Treft RL, Hillman T, Kennedy RJ, et al. Dominant optic atrophy, sensorineural hearing loss, ptosis, and ophthalmoplegia: a syndrome caused by a missense mutation in OPA1. Am J Ophthalmol. 2004;138(5):749–55. doi:10.1016/j.ajo.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 50.Ranieri M, Del Bo R, Bordoni A, Ronchi D, Colombo I, Riboldi G, Cosi A, Servida M, Magri F, Moggio M, et al. Optic atrophy plus phenotype due to mutations in the OPA1 gene: two more Italian families. J Neurol Sci. 2012;315(1–2):146–49. doi:10.1016/j.jns.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]