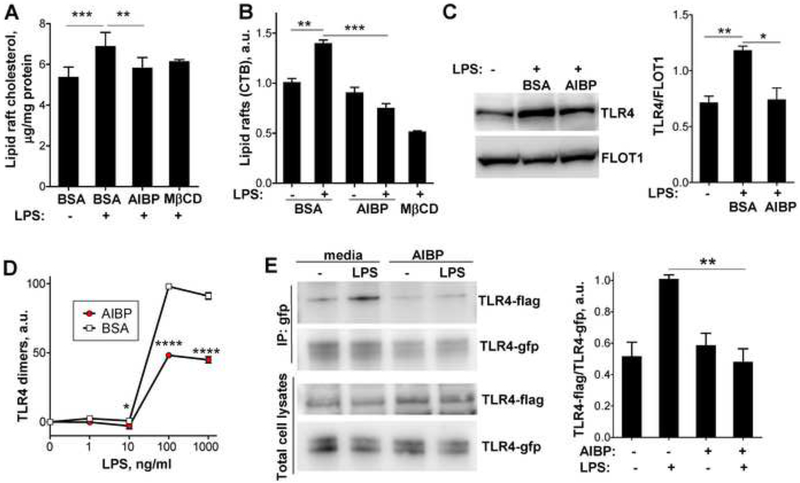
Figure 2. AIBP disrupts lipid rafts and inhibits TLR4 dimerization.
A-C, BV-2 cells were incubated for 2 hours with vehicle (0.1% BSA) or 0.2 μg/ml AIBP (in 0.1% BSA) in serum-containing medium and stimulated with 10 ng/ml LPS for 10 min. A, Content of free cholesterol in isolated raft fractions was normalized to total cell protein. Mean±SEM; n=6 for first 3 columns; ***, p<0.001; **, p<0.01 (repeated measures ANOVA; raft isolation was performed for a single replicate of all samples per day); n=3 for MβCD. Mean±SEM; n=3; ***, p<0.001; **, p<0.01 (one-way ANOVA). B, Content of CTB-positive lipid rafts was measured in a flow cytometry assay. C, TLR4 occupancy in isolated lipid rafts was tested in western blot. Mean±SEM; n=3; **, p<0.01; *, p<0.05 (one-way ANOVA). D, BV-2 cells were preincubated for 2 h with 0.2 μg/ml BSA or AIBP, followed by a 15 min incubation with LPS. Arbitrary numbers of TLR4 dimers were measured in a FACS assay with MTS510 and SA15–21 TLR4 antibodies as described in Methods. Mean±SD; n=3; p<0.05; ****, p<0.0001 (two-way ANOVA with Bonferroni post-test). E, Ba/F3 cells stably expressing TLR4-gfp, TLR4-flag and MD2 were incubated with serum-free media containing 50 μg/ml HDL, in the presence or absence of 0.2 μg/ml AIBP, and then stimulated with 10 ng/ml LPS for 20 min. Cell lysates were immunoprecipitated with an anti-GFP antibody and blots were probed with anti-flag and anti-GFP antibodies. Mean±SEM; n=4–6; **, p<0.01; Student’s t-test. See also Figures S1B and S2.