Figure 7 |. Model for the bifunctional role of the UBL domain in UHRF1 ubiquitylation of histone H3.
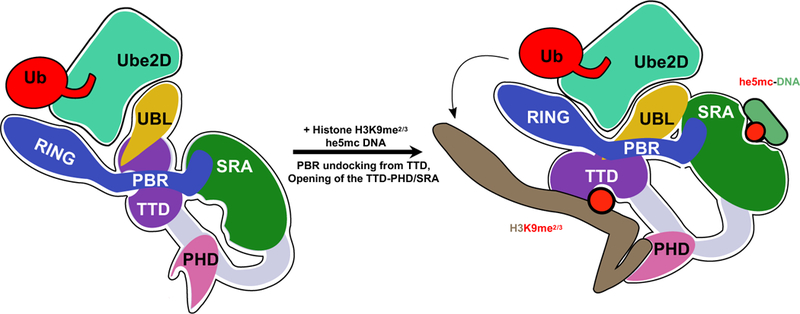
In the absence of chromatin (left), UHRF1 adopts a condensed conformation in which the histone- and DNA-binding modules interact and the linker that connects the SRA and RING (poly-basic region; PBR) occupies the histone-binding site of the TTD. In this state, both the UBL and RING domains can interact with E2~Ub, as judged by ITC and auto-ubiquitylation (Harrison et al., 2016a). Binding to histone or DNA, opens the protein allowing for high-affinity interactions with chromatin. However, productive H3 ubiquitylation is only achieved when he5mc DNA is bound to the SRA (right). The UBL recruits Ube2D, and also positions H3 near the active-site of the E2 through inter-domain interactions with UHRF1. Our cross-linking analysis indicates that the SRA domain is in close proximity to the UBL.
