Figure 3.
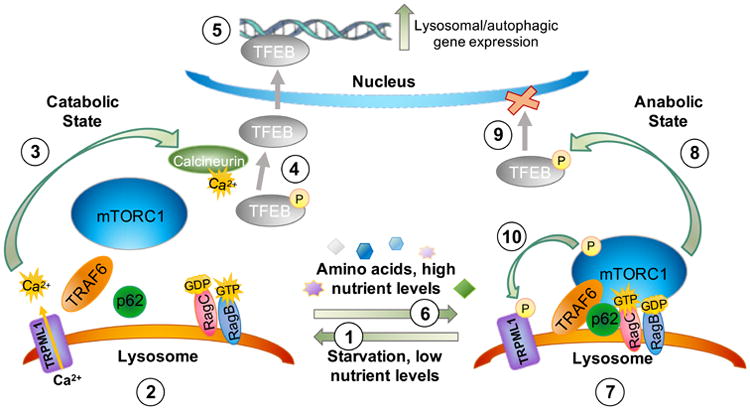
Role of mucolipin-1 (TRPML1) during mTOR and TFEB signaling. As described in the text, in response to a low nutrient state (1) mTOR dissociates from the lysosome and lysosomal calcium efflux is triggered through mucolipin-1 (2), allowing for activation of the phosphatase calcineurin (3). Calcineurin in turn dephosphorylates TFEB (4), which promotes its translocation across the nuclear envelope to activate lysosomal biogenesis and autophagy gene expression (5). In response to a high nutrient state (6), mTORC1 scaffolding proteins are recruited to the lysosomal surface and mTORC1 docks on this lysosomal platform (7). Activated mTORC1 then phosphorylates TFEB (8), which promotes its association with 14-3-3 proteins sequestering TFEB within its cytosolic location and thereby downregulates lysosomal biogenesis/autophagy gene expression (9). In response to nutrient availability, mTORC1 also phosphorylates mucolipin-1 directly (10), which reduces its channel activity and would further promote cytosolic localization of TFEB in a phosphorylated state. Mucolipin-1's ability to respond to low nutrient states by releasing calcium (see 2, above) has been suggested to be due to relief of mTOR-mediated phosphorylation of mucolipin-1. These key roles of mucolipin-1 in the nutrient sensing pathway governed by mTORC1 have been previously highlighted in other reviews (Venkatachalam et al. 2013). (Medina et al. 2015; Wang et al. 2015; Onyenwoke et al. 2015; Roczniak-Ferguson et al. 2012; Settembre et al. 2013a).
