Figure 2 |. Physics at the microscale.
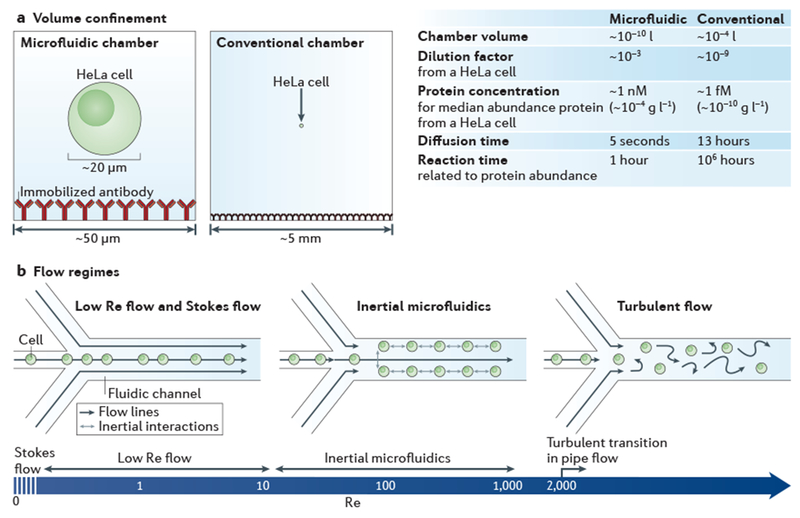
a | Bioanalysis in small, confined microfluidic volumes can enhance reaction speeds for detecting low-abundance molecules, compared with reactions conducted in a conventional chamber. In the case of detecting proteins from a single HeLa cell (median protein abundance ~170,000 molecules per cell27), a sufficient protein concentration is maintained in the microfluidic system for an antibody (assuming kon = 105 M−1 s−1 and a 60 kDa protein) to reach reaction equilibrium within ~1 hour, whereas it would take ~106 hours to reach equilibrium in the conventional system, owing to the relative concentration differences. The minimal diffusive losses that are associated with microfluidic volumes are essential for measuring proteins from single cells and other analytes that cannot be amplified, b | The predictable laminar flow that occurs in a microfluidic device is one of its most important features. The hydrodynamic flow focusing of cells is shown for different flow regimes, which vary according to the flow velocity, channel diameter or viscosity (these parameters are reflected in the Reynolds number (Re)). At near Stokes flow (Re <<1) and low Re flow (Re <10), which are the conditions in most microfluidic devices, the effects of inertial forces are minimal and for most applications can be ignored. In inertial microfluidics (typically 10 < Re <1,000), high pressures are used to create relatively high flow velocities, so that particles (such as cells) have inertial interactions with objects or flows. Inertial microfluidics can be used for several different applications145, including size-based sorting, streamline focusing and mechanical measurements12. Above a Re of 2,000 in pipe flow146, which is not practical to achieve in microfluidic devices, a transition to turbulent flow occurs.
