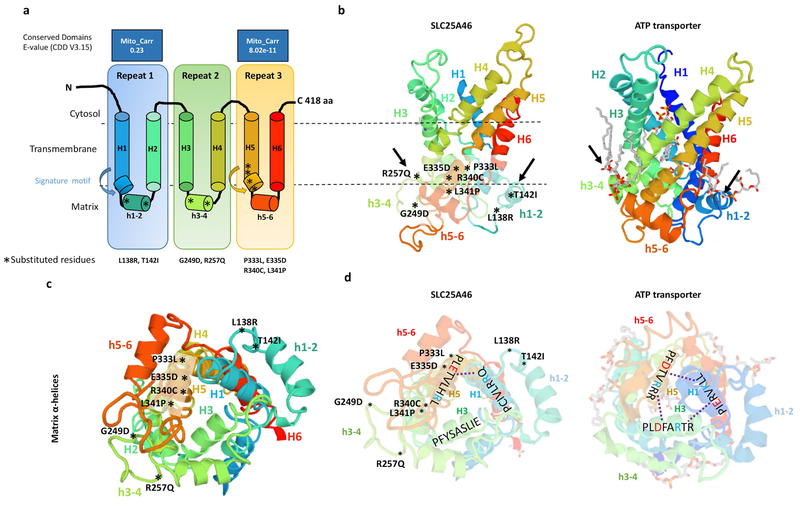
Figure 5. Predicted model of the SLC25A46 protein with the relative position of all reported point mutations.
(a) Topology of SLC25A46 inferred from homology to the mitochondrial carrier family. (b) Predicted 3D model of SLC25A46 based on threading to the crystal structure of the ATP transporter depicted to the right. Note the lipid chains in gray associated with the h3–4 and h1–2 helices of the ATP transporter, which are close to the relative position of the p.R257Q, p.G249D, p.L138R, and p.T142I mutations in SLC25A46. (c) SLC25A46 3D structure rotated to expose the matrix pore. The p.P333L, p.E335D, p.R340C, and p.P341L variants occur in the signature motif of H5, while the p.G249D, p.R257Q, p.L138R, and p.T142I occur in the minor h3–4 and h1–2 helices far away from the pore. (d) In the ATP transporter the (PX[D/E]XX[K/R]X[K/R]) signature motifs form salt bridges (dashed line) between the [D/E] and [K/R] residues between helixes 1,3,5. In SLC25A46 the signature motif is absent in H3 and most of the salt bridges are not predicted to form.