Figure 1. A model for A-ring aromatic steroid synthesis pathway in the octocoral cnidarian Dendronephthya studeri, based on the molecules described in Yan et al., 2011, and the knowledge of enzymatic pathways known from other organisms.
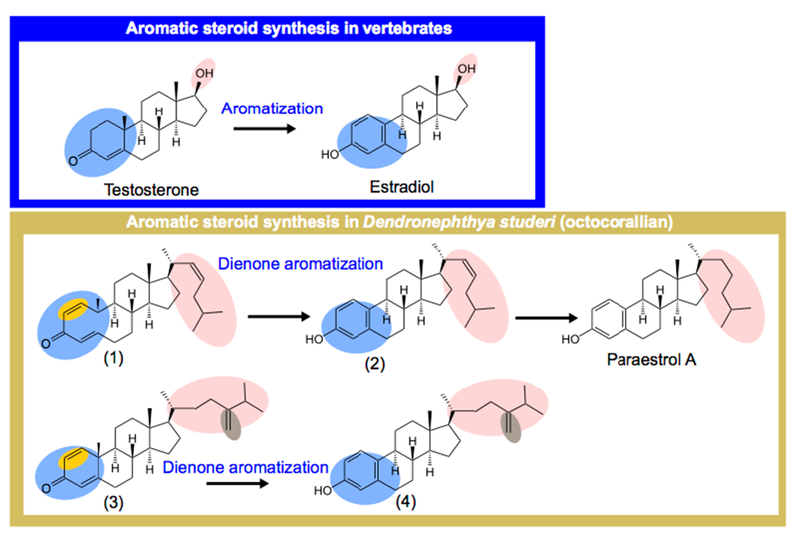
(1): Cholesta-1,4,22-trien-3-one; (2): (22E)-19-Norcholesta-1,3,5(10),22-tetraen-3-ol; (3): 24-Methylenecholesta-1,4,22-trien-3-one; (4): 24-Methylene-19-norcholesta-1,3,5(10),22-tetraen-3-ol. Paraestrol A (19-norcholesta-1,3,5(10)-trien-3-ol) has been proposed as an ancestral steroid [28]. We have named « dienone aromatization » the proposed aromatization reaction to stress the difference from the vertebrate case where there is no delta1–2 double bond (highlighted in yellow) on the A ring.
