Figure 4. Paraestrol A docking into the ligand binding pocket of an estrogen receptor a homology-based model of Hydra NR3E.
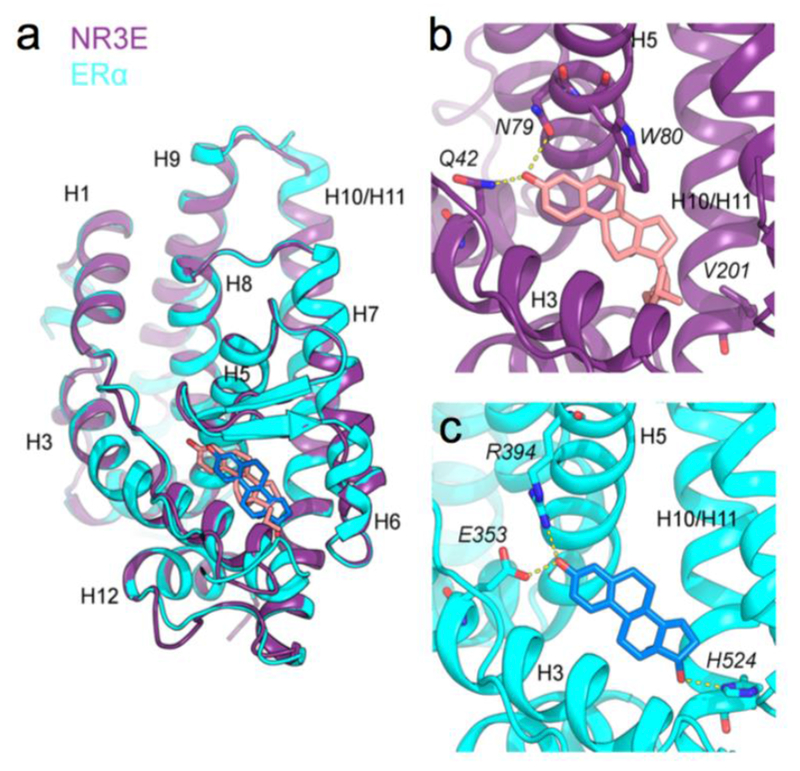
(a) Superposition of the NR3E structural model using ERα structure (in violet) where the paraestrol A is represented in pink, the ERα structure used as template (PDB id: 1ERE) is represented in cyan and the 17β-estradiol in blue, (b) Detailed view of the binding pocket of the NR3E ERα-based model, highlighting interactions (in yellow dashed lines) between the paraestrol and residues Q42 and N79. W80 involved in the cnidarian-specific π-π interaction is also shown, (c) Detailed view of the binding pocket of ERα, highlighting interactions (in yellow dashed lines) between the 17β-estradiol and residues E353, R394 and H524 (corresponding to V201 in NR3E model).
