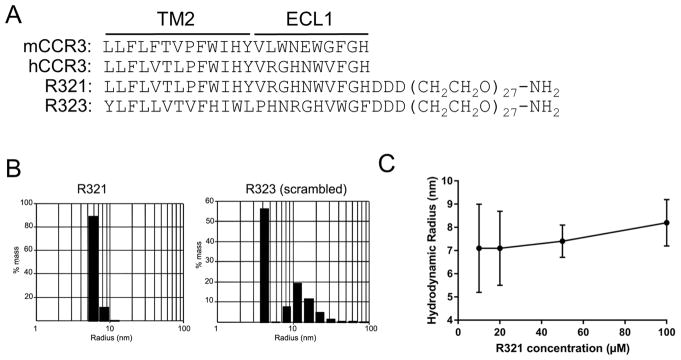
Figure 1. The R321 CCR3 peptide and its scrambled control (R323) self-assemble into nanoparticles.
(A) Structures of R321 and the scrambled peptide R323. Alignment with human and mouse CCR3 shows a high degree of identity at the TM2 region. (B) Dynamic Light Scattering (DLS) regularization distribution histograms are shown for 10 μM peptide solutions in PBS. Radii for R321 and R323 are 7.1 ± 0.7 nm and 4.5 ± 0.4 nm, respectively, with R323 somewhat smaller and more polydisperse; the polydispersity index of R321 and R323 were 0.07 and 0.28, respectively. Results represent mean ± SEM from experiments (n=3) performed in duplicate (C). R321 self-assembly into nanoparticles shows no dependence on peptide concentration. TM: transmembrane. ECL: extracellular loop.