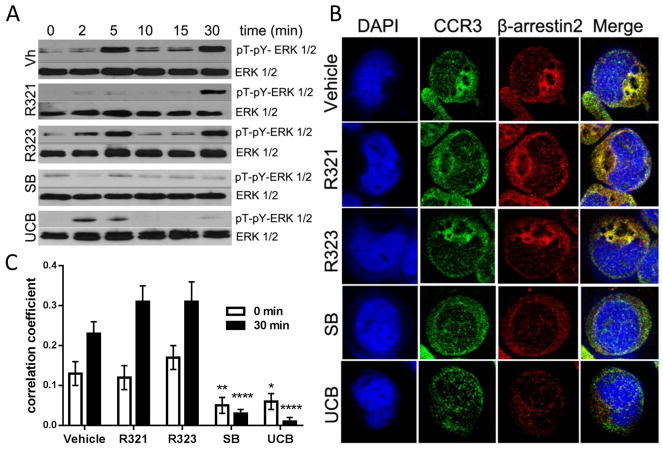
Figure 4. R321 does not inhibit ligand-induced β-arrestin recruitment and signaling by activated CCR3.
(A) Following CCR3 activation with 100 nM CCL11, biphasic ERK1/2 phosphorylation was observed. Acute (2–5min) phosphorylation is mediated by G protein signaling. Late phase (30min) phosphorylation is likely due to β-arrestin signaling. R321 (10μM) inhibits only acute phosphorylation of ERK1/2. Scrambled peptide control – R323 (10μM) does not inhibit acute or late phase phosphorylation. SB328437 (10μM) inhibits both acute and late phase phosphorylation and UCB35625 (10μM) inhibits the late phase to a higher degree than the early phase. (B) Representative confocal images of AML14.3D10-CCR3 cells exposed to vehicle or inhibitors for 30 min and stimulated with CCL11/eotaxin-1 for 30 min. (C) Quantitation by Pearson’s correlation method shows colocalization of CCR3 to β-arrestin2 30 min after stimulation with CCL11/eotaxin-1. R321 and R323 (10 μM) did not inhibit CCL11-induced β-arrestin2 recruitment to CCR3 whereas the CCR3 antagonist SB328437 and UCB35625 strongly inhibited colocalization. Results represent mean (50 cells per treatment group) ± SEM from 3 independent experiments. (*p ≤ 0.05, **p≤ 0.01,****p ≤ 0.0001 as compared to control).