Figure 3. A putative roadmap to more personalized CRC management based on molecular subtyping.
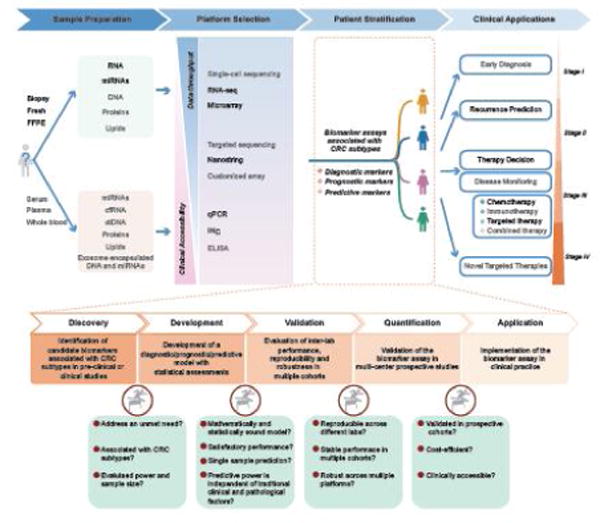
Implementing CMS subtyping for more personalized clinical management of CRC patients involves four major phases: (1) Collection of pre- or post-surgical biopsies, surgical tissues or whole blood/serum/plasma from CRC patients to isolate DNA/RNA/miRNA and proteins to perform molecular profiling. (2) Performing transcriptomic as well as multi-omic profiling using various high and low throughput platforms that are currently available as well as the methods that are under development. (3) Stratifying patients by various biomarker assays tailored for specific clinical applications. More specifically, establishing a robust biomarker associated with CRC subtyping involves multiple stages: biomarker discovery, model development, inter-lab validation as well as validation by prospective studies. At each stage, critical assessments of the assay are needed before entering the next stage. (4) Implementation of subtyping in decision making of CRC to address various clinical questions throughout the CRC progression. Multiple choices of sample sources, molecules and profiling platforms are available, yet largely unexplored (colored in gray), for developing an optimized biomarker assay for a specific clinical application.
