Abstract
The current study tested the hypothesis that voluntary activation during maximal voluntary contraction (MVC) conditionally depends on sex and joint action. Twenty-eight healthy adults (14 of each sex) performed knee extensor MVC and plantar flexor MVC at extended and flexed knee positions. Voluntary activation during MVC was assessed using a twitch interpolation technique. The voluntary activation during plantar flexor MVC at the extended knee position was significantly lower (P = 0.020, 95% confidence interval 1.4 to 14.6, Cohen’s d for between-subject design = 0.94) in women (88.3% ± 10.0%) than in men (96.2% ± 6.6%). In contrast, no significant sex differences were shown in the voluntary activation during knee extensor MVC (93.7% ± 5.9% (women) vs. 95.0% ± 3.9% (men)) and during plantar flexor MVC at the flexed knee position (90.4% ± 12.2% (women) vs. 96.8% ± 5.6% (men)). The voluntary activation during knee extensor MVC was significantly higher (P = 0.001, 95% confidence interval 2.1 to 8.8, Cohen’s d for within-subject design = 0.69) than that during plantar flexor MVC at the extended knee position in women, whereas the corresponding difference was not observed in men. The results revealed that the existence of sex difference in the voluntary activation during MVC depends on joint action and joint angle.
Keywords: Knee extension, Plantar flexion, Sex difference, Twitch interpolation technique
Introduction
A magnitude of muscle activation during maximal voluntary isometric contractions (MVCs) is the major determinant of generated muscular force. Voluntary activation (VA%), determined by the twitch interpolation technique (Shield & Zhou, 2004), is an index often used to represent the magnitude of muscle activation.
It has been shown that the magnitude of VA% depends on movements at joints (joint action). For example, male participants demonstrated a lower VA% during knee extensor MVC than during plantar flexor MVC (Behm et al., 2002). As a possible reason for the difference, Behm et al. (2002) proposed the difference in the muscle fiber type compositions of agonist muscle groups, i.e., a difficulty in full recruitment of motor units during knee extensor MVC due to the relatively higher proportion of type II fibers of the quadriceps femoris compared with the triceps surae. The proportion of type II fibers of the vastus lateralis, the largest muscle among the quadriceps femoris in both sexes (Ema et al., 2017), is lower in women than in men (Hunter, 2014). Although the association between VA% and muscle fiber type composition is unclear, if the proposal by Behm et al. (2002) is correct, women may show a higher magnitude of VA% during knee extensor MVC than men. In contrast, the soleus, which has the largest physiological cross-sectional area among the triceps surae (Fukunaga et al., 1992), comprises mainly type I fibers even in men (Johnson et al., 1973), suggesting that any possible sex difference of the soleus fiber type composition will be smaller compared with the corresponding difference of the vastus lateralis. Moreover, given that knee flexion reduces the neural and mechanical contribution of the gastrocnemius to plantar flexion strength (Wakahara et al., 2007), it can be assumed that the sex difference in VA% during plantar flexor MVC, if any, will be small, especially at a flexed knee position. A training program induced a similar extent of strength gains between sexes despite a smaller magnitude of muscle hypertrophy in women (Lemmer et al., 2000; Melnyk, Rogers & Hurley, 2009), which may indicate greater neural adaptation by the training in women than in men. Considering that the magnitude of VA% before training intervention (Gondin et al., 2005) and its training-induced change (Ema, Saito & Akagi, 2018) were related to strength gain, an investigation of the above notions should promote better understanding of the underpinning mechanisms of training-induced strength improvement in men and women.
To the best of our knowledge, no studies have investigated the effects of sex and joint action on VA% during MVC simultaneously. In the current study, we determined VA% during knee extensor MVC and plantar flexor MVC at extended and flexed knee positions in both sexes. We tested the hypothesis that VA% during MVC conditionally depends on sex and joint action.
Methods
Participants
A sample size estimation (G*Power 3.1.7, Kiel University, Germany) was performed to detect a within-between interaction for VA%. The expected effect size, α, power, and correlation among repeated measures were set at 0.25, 0.05, 0.80, and 0.5, respectively. The estimation showed that 28 participants are required. It was proposed that physical activity and the existence of practice for strength testing affect the magnitude of VA% (Hunter, Pereira & Keenan, 2016). Therefore, we recruited untrained healthy young adults, and all participants visited our laboratory in advance for familiarization and practice in performing MVCs with the experimental setting for the right leg. A total of 28 adults (14 of each sex) with no habitual resistance exercises, knee or ankle injuries participated in the study (Table 1). We confirmed no significant sex difference in the magnitude of habitual physical activity, assessed with the long version of the International Physical Activity Questionnaire (Craig et al., 2003). The strength testing for knee extension and plantar flexion was performed on different days in random order among the participants. This study was approved by the Ethics Committee of the Shibaura Institute of Technology (Acceptance number: 16-008). All participants were informed of potential risks and the study’s purpose, and they provided written informed consent before participation.
Table 1. Physical characteristics of participants.
| Men (n = 14) | Women (n = 14) | ||
|---|---|---|---|
| Age | years | 23 ± 4 | 22 ± 1 |
| Height | cm | 169.7 ± 4.4 | 157.6 ± 4.1 |
| Body mass | kg | 62.0 ± 6.2 | 51.5 ± 6.6 |
| Physical activity | MET min/wk | 3,108 ± 2,177 | 2,836 ± 2,542 |
Notes.
MET, metabolic equivalent. Data are shown as mean ± standard deviation.
Evoked twitch responses
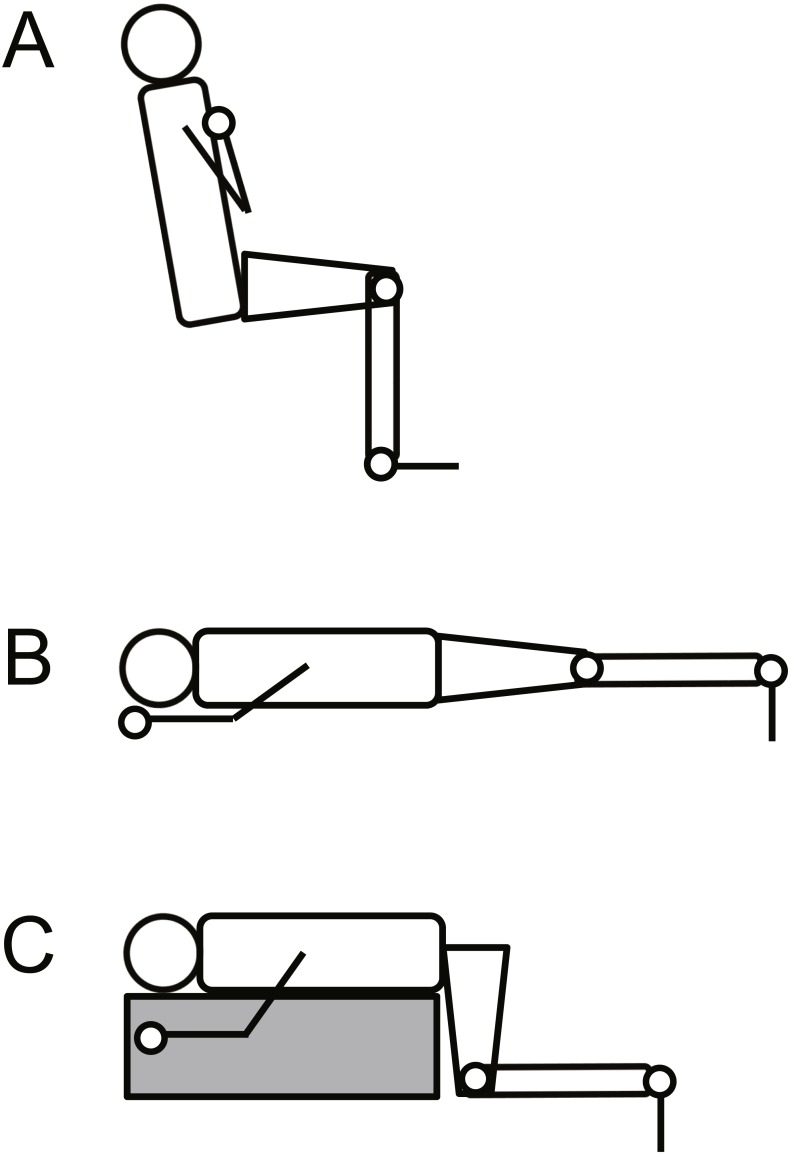
To provide insights into the effects of muscle fiber type composition on VA%, we investigated the twitch contractile properties, because the properties have been reported to be associated with the composition (Harridge et al., 1996; Hamada et al., 2000). Participants sat (for knee extensions) or lay supine (for plantar flexions) on the bench of an isokinetic dynamometer (CON-TREX MJ, PHYSIOMED, Germany) while being secured at the pelvis and torso to the dynamometer with nonelastic straps (Fig. 1). The knee and hip joint angles were set at 90° and 80°, respectively, for knee extension (anatomical position = 0°). For plantar flexion, the knee joint angle was 0° (K0) or 90° (K90) and the ankle joint angle was 0° (Kennedy & Cresswell, 2001). The centers of rotation of the dynamometer and the right knee/ankle joints were visually adjusted. Using a constant current variable voltage stimulator (DS7A; Digitimer Ltd, Welwyn Garden City, UK), the quadriceps femoris and triceps surae twitch responses were obtained with rectangular pulses of 1 ms. For the quadriceps femoris, to percutaneously stimulate the femoral nerve, a cathode (2 × 2 cm) was placed in the femoral triangle, and an anode (4 × 5 cm) was placed in midway between the superior aspect of the greater trochanter and the inferior border of the iliac crest. For the triceps surae, the tibial nerve was stimulated percutaneously in the popliteal fossa with the cathode and over the ventral aspect of the thigh with the anode. The supramaximal stimulus intensity was determined by increasing the current intensity until plateaus in the twitch torque occurred. Thereafter, five supramaximal twitch responses at a higher current (≥20%) were obtained every 10 s. Torque signals were recorded at 4 kHz and stored in a personal computer after A/D conversion (PowerLab16/35; ADInstruments, Bella Vista, Australia). After low-pass filtering the signal at 500 Hz, contraction onset was manually identified as described previously (Ema, Saito & Akagi, 2018). A previous study used the time to peak twitch torque (TPT), i.e., the duration from torque onset to peak twitch torque, as an index of estimated muscle fiber type composition (Kubo & Ikebukuro, 2010). Moreover, TPT was associated with muscle fiber type composition (Hamada et al., 2000). However, TPT is possible to depend on the magnitude of the peak value of twitch torque, making it unsuitable for comparisonsbetween sexes and between different joint actions. Therefore, we determined the twitch torque at 50 ms from torque onset relative to the peak value of twitch torque (normalized Twitch0−50) (Balshaw et al., 2016), and used this metric as the index of estimated muscle fiber type composition. The data were averaged across five contractions.
Figure 1. Schematic illustration of the experimental setup for knee extension (A), plantar flexion at extended (B) and at flexed knee positions (C).
VA% evaluations
After several warm-up contractions involving two maximal MVCs, participants performed knee extensor/plantar flexor MVCs for 3 s two times. A one-minute rest was provided between contractions. Verbal encouragement was provided during the contractions. When peak torque was visually observed on screen and 2 s after the MVC, supramaximal triplet stimulations at 100 Hz were interpolated. If the difference in the peak value of torque before stimulation was above 10%, an additional contraction was requested. The VA% was calculated as follows: (1 − [superimposed triplet torque/potentiated resting triplet torque]) ×100 (Miyamoto et al., 2012). The mean of the two trials was used for subsequent analyses.
Statistical analyses
Statistical analyses were performed using SPSS version 22 (IBM, Armonk, NY, USA). All data are shown as means ± standard deviation. The significance level was set at P < 0.05. A two-way analysis of variance (ANOVA) with one between-group factor (sex; men and women) and one within-group factor (joint action; knee extension, plantar flexion in K0 and K90) was conducted on dependent variables. When a significant interaction of sex × joint action was shown, follow-up ANOVAs with Bonferroni multiple-comparisons were used. To examine the magnitude of the difference in variables, Cohen’s d (between- or within-subject designs, (Lakens, 2013) was calculated as an index of effect size (ES), and 95% confidence interval of the difference were determined. The thresholds for interpretation of ES were 0.20, 0.60, and 1.20 for small, medium, and large (Hopkins et al., 2009). We considered the differences to be substantial if both ES >0.60 and P <0.05.
Results
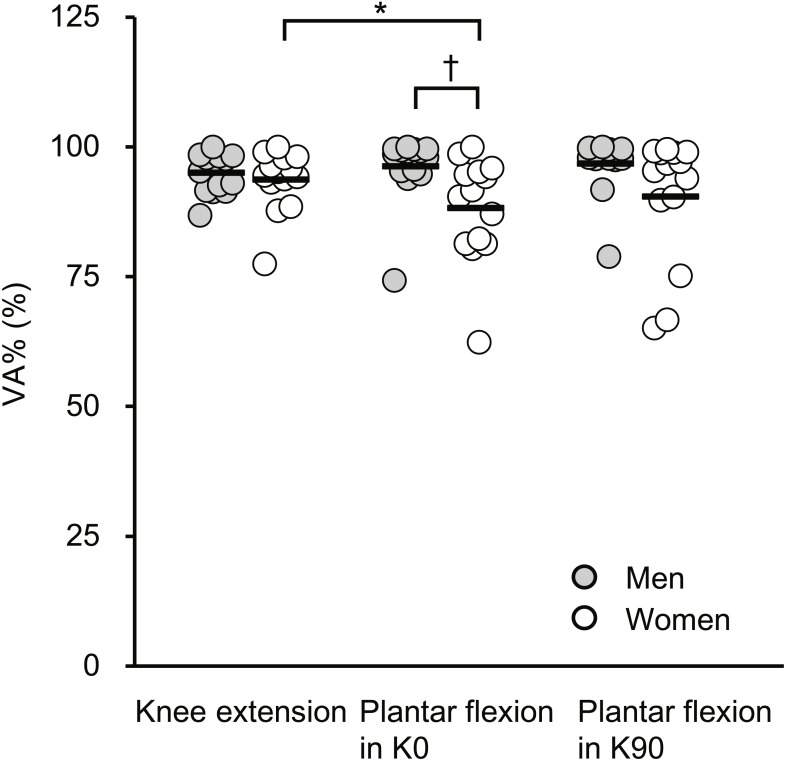
Figure 2 shows VA% during MVC. There was a significant sex × joint-action interaction (F[2, 52] = 3.221, P = 0.048) and significant main effect of sex (F[1, 26] = 4.27, P = 0.049) but not of joint action (F[2, 52] = 1.198, P = 0.310). Regarding the sex difference, VA% during plantar flexor MVC in K0 was significantly higher in men (96.2% ± 6.6%) than in women (88.3% ± 10.0%), whereas no significant sex difference was found for VA% during knee extensor MVC (95.0% ± 3.9% [men] vs. 93.7% ± 5.9% [women]) or plantar flexor MVC in K90 (96.8% ± 5.6% [men] vs. 90.4% ± 12.2% [women]), with the observed effects as small to medium (Table 2). For joint action dependency, VA% during knee extensor MVC was significantly higher than that during plantar flexor MVC in K0 in women, with the effect being medium, but not in men. No corresponding differences were shown between other joint actions in each sex.
Figure 2. Scatterplots showing individual data of voluntary activation (VA%) during maximal voluntary contractions of knee extension, plantar flexion at extended (K0) and at flexed (K90) knee positions.
Solid line shows the mean value. *Indicates a significant difference between joint actions. † Shows a significant difference between sexes.
Table 2. Statistical results of the difference between sexes and between joint actions.
| P value | ES | 95% confidence interval | |
|---|---|---|---|
| VA% | |||
| Males vs. Females | |||
| KE | 0.501 | 0.26 | −2.6 to 5.1 |
| PF in K0 | 0.020 | 0.94 | 1.4 to 14.6 |
| PF in K90 | 0.086 | 0.68 | −1.0 to 13.8 |
| KE vs. PF in K0 | |||
| Males | 0.421 | 0.24 | −4.4 to 1.9 |
| Females | 0.001 | 0.69 | 2.1 to 8.8 |
| KE vs. PF in K90 | |||
| Males | 0.418 | 0.39 | −4.6 to 0.9 |
| Females | 0.152 | 0.36 | −2.9 to 9.5 |
| PF in K0 vs. in K90 | |||
| Males | 0.772 | 0.10 | −1.9 to 0.7 |
| Females | 0.299 | 0.19 | −8.2 to 3.9 |
| Normalized twitch0−50 | |||
| Males vs. Females | |||
| KE | 0.545 | 0.25 | −5.6 to 2.8 |
| PF in K0 | 0.545 | 0.08 | −3.3 to 4.0 |
| PF in K90 | 0.545 | 0.33 | −6.4 to 2.6 |
| KE vs. PF in K0 | |||
| Males | <0.001 | 2.00 | 8.3 to 15.1 |
| Females | <0.001 | 3.37 | 10.1 to 16.7 |
| KE vs. PF in K90 | |||
| Males | <0.001 | 1.87 | 8.0 to 16.8 |
| Females | <0.001 | 2.80 | 8.3 to 15.4 |
| PF in K0 vs. in K90 | |||
| Males | 1.000 | 0.12 | −1.4 to 2.7 |
| Females | 1.000 | 0.35 | −3.5 to 0.4 |
Notes.
- ES
- Cohen’s d for between- or within-subject designs
- KE
- knee extension
- Normalized twitch0−50
- twitch torque at 50 ms from torque onset relative to the peak value of twitch torque
- PF in K0
- plantar flexion at extended knee position
- PF in K90
- plantar flexion at flexed knee position
- VA%
- voluntary activation
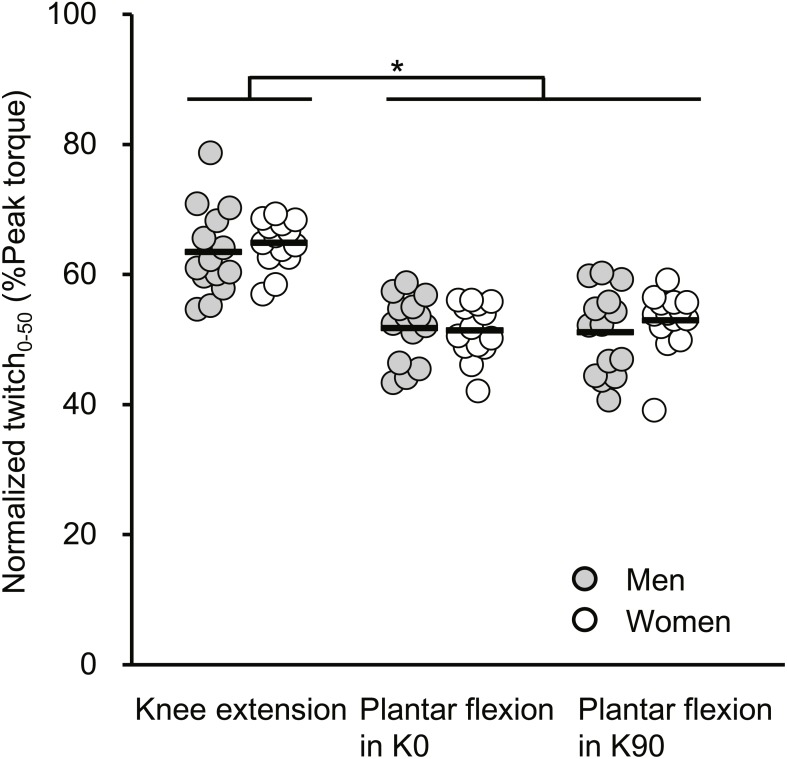
The normalized Twitch 0−50 results are described in Fig. 3. A significant main effect of joint action (F[2, 52] = 90.321, P < 0.001) without a main effect of sex (F[1, 26] = 0.376, P = 0.545) or an interaction of the two factors (F[2, 52] = 0.607, P = 0.549, partial η2 = 0.023) was shown. The normalized Twitch 0−50 of knee extension was significantly greater than those of plantar flexions for both extended and flexed knee positions, with the effects being large.
Figure 3. Scatterplots showing individual data of twitch torque at 50 ms from torque onset relative to the peak value of twitch torque (Normalized twitch0−50) of knee extension, plantar flexion at extended (K0) and at flexed (K90) knee positions.
Solid line shows the mean value. *Indicates a significant difference between joint actions.
Discussion
The main finding of the current study was that the substantial sex difference in VA% was shown only during plantar flexor MVC at the extended knee position. Compared with men, women showed lower VA% during plantar flexor MVC at the extended knee position, and the magnitude of sex difference was interpreted as medium (ES = 0.94). In contrast, a clear sex difference in VA% was not observed during knee extensor MVC or during plantar flexor MVC at the flexed knee position. In addition, in women but not in men, VA% during plantar flexor MVC at the extended knee position was substantially different from that during knee extensor MVC. These results indicate that the sex difference in VA% during MVC depended on joint action.
The substantial sex difference in VA% was shown only during plantar flexor MVC in K0. The normalized Twitch0−50 was not significantly different between sexes for any joint action (Fig. 2), suggesting that the muscle fiber type composition is not a major factor for the observed sex and joint-action dependency in VA%. Co-contraction of the antagonist tibialis anterior during plantar flexor MVC might be the explainable factor for the current result. It was shown that compared with males, females exhibited higher magnitude of the tibialis anterior activation relative to medial gastrocnemius activation during the push-off phase of countermovement jumping (Márquez et al., 2017). During countermovement jumping, just before take-off, the fascicles of the medial gastrocnemius have been reported to contract quasi-isometrically (Kurokawa et al., 2003); therefore, the contraction type of the triceps surae may be partly similar between the current (i.e., isometric contraction) and previous (Márquez et al., 2017) studies. Because of reciprocal inhibition (Crone et al., 1987), co-contraction of the tibialis anterior during plantar flexor MVC could diminish the magnitude of triceps surae activation during plantar flexion. In contrast, the absence of a clear sex difference in VA% during knee extensor MVC (Fig. 2) is in line with previous studies (Krishnan & Williams, 2009; Lee et al., 2017), and Krishnan & Williams (2009) observed that the magnitude of hamstring activation as antagonists during knee extensor MVC was not different between sexes. Such notions may be also related to the substantially higher VA% during knee extensor MVC than during plantar flexor MVC in K0 only in women. Taken together, a possible sex difference in the antagonist activation may account for the current sex- and joint-action differences in VA%.
The lack of significant difference in VA% between joint actions in male participants is not consistent with the previous finding of a higher value of VA% during plantar flexor MVC than during knee extensor MVC in men (Behm et al., 2002). This discrepancy may be related to the difference in participant backgrounds. We recruited untrained participants because resistance training can affect the magnitude of VA% (Ema, Saito & Akagi, 2018). In contrast, Behm et al. (2002) examined subjects who participated in habitual resistance exercises or competitive sport activities. Although the kind of resistance training that the subjects had performed was not mentioned, it is possible that the effects of training on VA% during MVC differed between joint actions, likely resulting in the higher VA% during plantar flexor MVC than during knee extensor MVC (Behm et al., 2002).
Our findings may provide some implication regarding the training-induced strength gains. Previous studies demonstrated that greater muscle hypertrophy in men than in women after resistance training were accompanied by a lack of sex difference in knee extension strength gains (Lemmer et al., 2000; Melnyk, Rogers & Hurley, 2009). The previous results imply a greater neural adaptation in women than in men, because there was a negative correlation between VA% before training and the magnitude of strength improvement (Gondin et al., 2005). However, no significant sex difference in VA% during knee extensor MVC was found in the present study; therefore, it is difficult to explain the aforementioned results in terms of a sex difference in neural adaptations. In contrast, our data might suggest the greater training-induced increase in plantar flexion strength in women than in men, and the greater strength gains in plantar flexion than in knee extension in women; future attempts are required to clarify this subject.
Conclusion
The substantial sex difference in VA% was limited during plantar flexor MVC at the extended knee position, and only women showed joint action dependency in VA%. These results revealed that the existence of sex difference in VA% during MVC depends on joint action and joint angle.
Supplemental Information
Funding Statement
This work was supported in part by the grant from the Nakatomi Foundation and JSPS KAKENHI Grant Numbers JP16K01671/JP16H05918. There was no additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Ryoichi Ema conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Momoka Suzuki, Emi Kawaguchi and Itaru Saito performed the experiments, analyzed the data, authored or reviewed drafts of the paper, approved the final draft.
Ryota Akagi conceived and designed the experiments, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
This study was approved by the Ethics Committee of the Shibaura Institute of Technology (Acceptance number: 16-008).
Data Availability
The following information was supplied regarding data availability:
The raw data is included in Figs. 2 and 3 and the Supplemental File.
References
- Balshaw et al. (2016).Balshaw TG, Massey GJ, Maden-Wilkinson TM, Tillin NA, Folland JP. Training-specific functional, neural, and hypertrophic adaptations to explosive- vs. sustained-contraction strength training. Journal of Applied Physiology. 2016;120:1364–1373. doi: 10.1152/japplphysiol.00091.2016. [DOI] [PubMed] [Google Scholar]
- Behm et al. (2002).Behm DG, Whittle J, Button D, Power K. Intermuscle differences in activation. Muscle and Nerve. 2002;25:236–243. doi: 10.1002/mus.10008. [DOI] [PubMed] [Google Scholar]
- Craig et al. (2003).Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Medicine and Science in Sports and Exercise. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Crone et al. (1987).Crone C, Hultborn H, Jespersen B, Nielsen J. Reciprocal Ia inhibition between ankle flexors and extensors in man. Journal de Physiologie. 1987;389:163–185. doi: 10.1113/jphysiol.1987.sp016652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema, Saito & Akagi (2018).Ema R, Saito I, Akagi R. Neuromuscular adaptations induced by adjacent joint training. Scandinavian Journal of Medicine & Science in Sports. 2018;28:947–960. doi: 10.1111/sms.13008. [DOI] [PubMed] [Google Scholar]
- Ema et al. (2017).Ema R, Wakahara T, Hirayama K, Kawakami Y. Effect of knee alignment on the quadriceps femoris muscularity: cross-sectional comparison of trained versus untrained individuals in both sexes. PLOS ONE. 2017;12:e0183148. doi: 10.1371/journal.pone.0183148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga et al. (1992).Fukunaga T, Roy RR, Shellock FG, Hodgson JA, Day MK, Lee PL, Kwong-Fu H, Edgerton VR. Physiological cross-sectional area of human leg muscles based on magnetic resonance imaging. Journal of Orthopaedic Research. 1992;10:928–934. doi: 10.1002/jor.1100100623. [DOI] [PubMed] [Google Scholar]
- Gondin et al. (2005).Gondin J, Guette M, Ballay Y, Martin A. Electromyostimulation training effects on neural drive and muscle architecture. Medicine and Science in Sports and Exercise. 2005;37:1291–1299. doi: 10.1249/01.mss.0000175090.49048.41. [DOI] [PubMed] [Google Scholar]
- Hamada et al. (2000).Hamada T, Sale DG, MacDougall JD, Tarnopolsky MA. Postactivation potentiation, fiber type, and twitch contraction time in human knee extensor muscles. Journal of Applied Physiology. 2000;88:2131–2137. doi: 10.1152/jappl.2000.88.6.2131. [DOI] [PubMed] [Google Scholar]
- Harridge et al. (1996).Harridge SD, Bottinelli R, Canepari M, Pellegrino MA, Reggiani C, Esbjörnsson M, Saltin B. Whole-muscle and single-fibre contractile properties and myosin heavy chain isoforms in humans. Pflügers Archiv—European Journal of Physiology. 1996;432:913–920. doi: 10.1007/s004240050215. [DOI] [PubMed] [Google Scholar]
- Hopkins et al. (2009).Hopkins WG, Marshall SW, Batterham AM, Hanin J. Progressive statistics for studies in sports medicine and exercise science. Medicine and Science in Sports and Exercise. 2009;41:3–13. doi: 10.1249/MSS.0b013e31818cb278. [DOI] [PubMed] [Google Scholar]
- Hunter (2014).Hunter SK. Sex differences in human fatigability: mechanisms and insight to physiological responses. Acta Physiologica. 2014;210:768–789. doi: 10.1111/apha.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, Pereira & Keenan (2016).Hunter SK, Pereira HM, Keenan KG. The aging neuromuscular system and motor performance. Journal of Applied Physiology. 2016;121:982–995. doi: 10.1152/japplphysiol.00475.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson et al. (1973).Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. Journal of the Neurological Sciences. 1973;18:111–129. doi: 10.1016/0022-510X(73)90023-3. [DOI] [PubMed] [Google Scholar]
- Kennedy & Cresswell (2001).Kennedy PM, Cresswell AG. The effect of muscle length on motor-unit recruitment during isometric plantar flexion in humans. Experimental Brain Research. 2001;137:58–64. doi: 10.1007/s002210000623. [DOI] [PubMed] [Google Scholar]
- Krishnan & Williams (2009).Krishnan C, Williams GN. Sex differences in quadriceps and hamstrings EMG-moment relationships. Medicine and Science in Sports and Exercise. 2009;41:1652–1660. doi: 10.1249/MSS.0b013e31819e8e5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo & Ikebukuro (2010).Kubo K, Ikebukuro T. Relationship between muscle fiber type and tendon properties in young males. Muscle and Nerve. 2010;42:127–129. doi: 10.1002/mus.21695. [DOI] [PubMed] [Google Scholar]
- Kurokawa et al. (2003).Kurokawa S, Fukunaga T, Nagano A, Fukashiro S. Interaction between fascicles and tendinous structures during counter movement jumping investigated in vivo. Journal of Applied Physiology. 2003;95:2306–2314. doi: 10.1152/japplphysiol.00219.2003. [DOI] [PubMed] [Google Scholar]
- Lakens (2013).Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Frontiers in Psychology. 2013;4 doi: 10.3389/fpsyg.2013.00863. Article 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. (2017).Lee A, Baxter J, Eischer C, Gage M, Hunter S, Yoon T. Sex differences in neuromuscular function after repeated eccentric contractions of the knee extensor muscles. European Journal of Applied Physiology and Occupational Physiology. 2017;117:1119–1130. doi: 10.1007/s00421-017-3599-8. [DOI] [PubMed] [Google Scholar]
- Lemmer et al. (2000).Lemmer JT, Hurlbut DE, Martel GF, Tracy BL, Ivey FM, Metter EJ, Fozard JL, Fleg JL, Hurley BF. Age and gender responses to strength training and detraining. Medicine and Science in Sports and Exercise. 2000;32:1505–1512. doi: 10.1097/00005768-200008000-00021. [DOI] [PubMed] [Google Scholar]
- Márquez et al. (2017).Márquez G, Alegre LM, Jaén D, Martin-Casado L, Aguado X. Sex differences in kinetic and neuromuscular control during jumping and landing. Journal of Musculoskeletal and Neuronal Interactions. 2017;17:409–416. [PMC free article] [PubMed] [Google Scholar]
- Melnyk, Rogers & Hurley (2009).Melnyk JA, Rogers MA, Hurley BF. Effects of strength training and detraining on regional muscle in young and older men and women. European Journal of Applied Physiology and Occupational Physiology. 2009;105:929–938. doi: 10.1007/s00421-008-0979-0. [DOI] [PubMed] [Google Scholar]
- Miyamoto et al. (2012).Miyamoto N, Fukutani A, Yanai T, Kawakami Y. Twitch potentiation after voluntary contraction and neuromuscular electrical stimulation at various frequencies in human quadriceps femoris. Muscle and Nerve. 2012;45:110–115. doi: 10.1002/mus.22259. [DOI] [PubMed] [Google Scholar]
- Shield & Zhou (2004).Shield A, Zhou S. Assessing voluntary muscle activation with the twitch interpolation technique. Sports Medicine. 2004;34:253–267. doi: 10.2165/00007256-200434040-00005. [DOI] [PubMed] [Google Scholar]
- Wakahara et al. (2007).Wakahara T, Kanehisa H, Kawakami Y, Fukunaga T. Fascicle behavior of medial gastrocnemius muscle in extended and flexed knee positions. Journal of Biomechanics. 2007;40:2291–2298. doi: 10.1016/j.jbiomech.2006.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data is included in Figs. 2 and 3 and the Supplemental File.