Abstract
Prolonged noise exposures presented at low to moderate intensities are often used to investigate neuroplastic changes in the central auditory pathway. A common assumption in many studies is that central auditory changes occur independent of any hearing loss or cochlear dysfunction. Since hearing loss from a long term noise exposure can only occur if the level of the noise exceeds a critical level, prolonged noise exposures that incrementally increase in intensity can be used to determine the critical level for any given species and noise spectrum. Here we used distortion product otoacoustic emissions (DPOAEs) to determine the critical level in male, inbred Sprague-Dawley rats exposed to a 16–20 kHz noise that increased from 45 to 92 dB SPL in 8 dB increments. DPOAE amplitudes were largely unaffected by noise presented at 60 dB SPL and below. However, DPOAEs within and above the frequency band of the exposures declined rapidly at noise intensities presented at 68 dB SPL and above. The largest and most rapid decline in DPOAE amplitude occurred at 30 kHz, nearly an octave above the 16–20 kHz exposure band. The rate of decline in DPOAE amplitude was 0.54 for every 1 dB increase in noise intensity. Using a linear regression calculation, the estimated critical level for 16–20 kHz noise was remarkably low, approximately 60 dB SPL. These results indicate that long duration, 16–20 kHz noise exposures in the 65–70 dB SPL range likely affect the cochlea and central auditory system of male Sprague-Dawley rats.
Keywords: Distortion product otoacoustic emissions, prolonged noise exposure, critical intensity, hearing loss
1. Introduction
Industrialized societies are becoming increasingly noisier, with city roadway noise often reaching average equivalent intensities of 75 dB SPL Leq (Barrigón Morillas et al., 2002; Kheirbek et al., 2014). The adverse effects of environmental noise can be compounded by long-duration exposures such as those that as occur on merchant or military ships and commercial airlines (Beierle, 1996; Buckey et al., 2001). Concerns about the effects of long term noise exposure have not only focused on the cochlea, but also the central nervous system. Recent studies performed in rats, cats, and mice have demonstrated that prolonged exposure to moderate intensity noise (≤75 dB SPL) can result in neuroplastic changes throughout the central auditory pathway (Lau et al., 2015; Pienkowski and Eggermont, 2009; Pienkowski and Eggermont, 2010; Sheppard et al., 2017; Zheng, 2012; Zhou and Merzenich, 2012). Furthermore, low-level noise is often used to treat tinnitus and hyperacusis, and has been found to alter human loudness perception (Formby et al., 2003; Henry et al., 2006; Norena and Chery-Croze, 2007).
Behavioral threshold shifts resulting from long-term noise exposures have been extensively studied in several species, including humans in the 1970s and 80s (Blakeslee et al., 1978; Melnick, 1991; Syka and Popelar, 1980). Audiometric thresholds progressively increased during the first 18–24 hours of the noise exposure; however, thresholds thereafter remained stable over weeks or months or even years (Carder and Miller, 1971; Carder and Miller, 1972). Prolonged exposures thus give rise to an asymptotic threshold shift (ATS). For noise exposure that are infinitely long, the ATS presumably estimates the maximum permanent threshold shift (PTS) that can develop from that particular exposure. If the noise exposure used to induce an ATS is of moderate intensity and lasts only a few days, then the hearing loss will largely recover, i.e., a temporary threshold shift (TTS) following the ATS. However, for moderately intense exposures lasting weeks or months, the ATS represents a combined threshold shift consisting of TTS and PTS as discussed in earlier publications (Carder et al., 1971; Carder et al., 1972; Mills, 1973). An ATS will only develop if the intensity (I, dB SPL) exceeds a critical level (C in dB SPL). The value of C varies across species and is dependent on frequency as well as other parameters of the noise exposure such as bandwidth (Blakeslee et al., 1978; Mills et al., 1978). The growth of ATS as a function of intensity increases at a constant rate (M=slope) for intensities greater than C. Thus, the hearing loss during a prolonged noise exposure can be predicted by the equation: ATS = M (I-C) where I represents the intensity of the noise and M represents slope or rate of growth of ATS per dB increase in the intensity above the critical level. In the chinchilla, ATS increases approximately 1.7 dB for every 1 dB increase in intensity above the critical level (Mills and Talo, 1972; Mills et al., 1979; Saunders et al., 1977). Depending on the intensity and duration of the prolonged exposure, the hearing loss can be temporary or permanent (Carder et al., 1972; Chen et al., 2014; Clark, 1991).
Long term noise exposures that incrementally increase in intensity can be used to identify noise levels that are ostensibly safe versus those that are potentially damaging to cochlear hair cells. The outer hair cells (OHCs), which enhance the sensitivity and frequency selectivity of the cochlea and which play a major role in determining audiometric thresholds, are substantially more vulnerable to acoustic overstimulation than inner hair cells (IHCs) (Saunders et al., 1991). Distortion product otoacoustic emissions (DPOAEs) generated by OHCs provide a sensitive, non-invasive functional measure for assessing OHC integrity (Wang et al., 1997). DPOAEs were used previously to distinguish safe versus deleterious sound intensities by exposing chinchillas to a series of consecutive 6-day, noise exposures (4 kHz octave band noise) that increased from 48 to 96 dB SPL in 8 dB steps (Eddins et al., 1999). DPOAEs started to decline above 50 dB SPL at the rate of 1.2 dB amplitude reduction per dB of noise intensity above the critical level; frequencies roughly a half octave above the noise were the most vulnerable. The DPOAE critical level was similar to those obtained from extremely time consuming behavioral measurements, which illustrates the utility of this approach (Mills et al., 1972). These results suggest that DPOAEs can be used to determine the minimal intensity capable of disrupting OHC function and auditory sensitivity.
We are currently using inbred Sprague-Dawley rats to investigate the neuroplastic changes occurring in the central auditory pathway following prolonged exposure to low-level noise. Our previous study measuring auditory brainstem responses (ABRs), suggested that the critical level in Sprague-Dawley rats was approximately 77 dB SPL (Chen et al., 2014). However, our more recent electrophysiological studies suggested that chronic exposure to low intensity noise might be affecting the cochlea at noise exposure intensities less than 77 dB SPL (Sheppard et al., 2017). To determine the minimum exposure intensity capable of affecting cochlear function, we carried out a noise dose-response study in which DPOAEs were measured in inbred Sprague-Dawley rats before, during, and after a series of 7-day noise exposures (16–20 kHz) that increased in intensity from 45 to 92 dB SPL in roughly 8 dB steps. Our results show that the critical level for this prolonged exposure is remarkably low, approximately 60 dB SPL; and that significant reductions in DPOAE amplitudes and thresholds occurred at noise exposure levels of 68 dB SPL.
2. Materials and Methods
2.1. Subjects:
Five male Sprague Dawley rats (3–6 months old, 300–400 g, Charles River) were used in the study. Animals were housed in the laboratory animal facility at the University at Buffalo and given free access to food and water ad libitum. Animals were maintained on a 12-hour light-dark cycle at a temperature of 22 °C. All experiments were approved by the Institutional Animal Care and Use Committee at the University at Buffalo.
2.2. Noise Exposure Protocol:
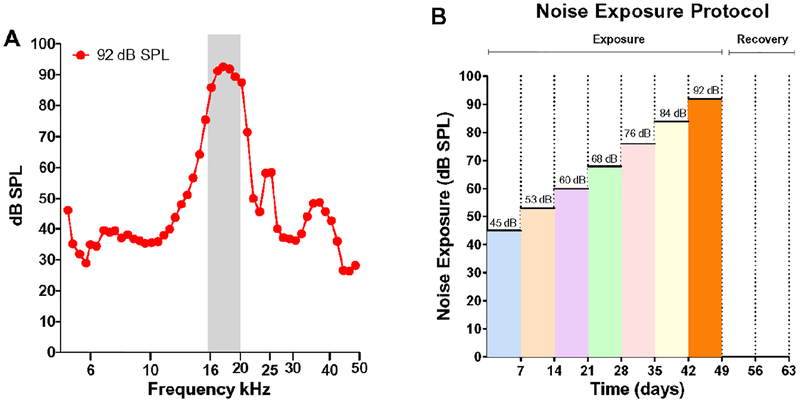
Animals were exposed 24h/d to 16–20 kHz narrowband noise (Figure 1A) starting at 45 dB SPL (maximum within band intensity); each week the intensity increased by ~8 dB SPL to a maximum of 92 dB SPL (Figure 1B). The noise was generated using Adobe Audition. The signal was generated through the PC soundcard (44 kHz sampling rate), fed to a power amplifier (Amp 300, AudioSource Inc.) and presented through a loudspeaker (Fostex FT17H) suspended approximately 8 cm above the acoustically transparent wire mesh ceiling of the acrylic cage (dimensions L = 48.2 cm, W = 25.4 cm, H = 20.3 cm) in which the rats were housed in the animal facility. Sound levels were measured at the height of animal’s ear directly below the speaker using a half-inch condenser microphone (Larson Davis; 2450), preamplifier and power supply (Larson Davis, 2221). The output of the microphone was digitized (RME, model Babyface Pro) and the acoustic signal was analyzed using custom MATLAB software. Figure 1A show the noise spectrum measured at 92 dB SPL. Sound levels at various locations within the cage varied by approximately +/− 2 dB SPL. During the first week of the exposure, the rats were exposed at 45 dB SPL; the level was progressively increased to 53, 60, 68, 76, 84, and 92 dB SPL in subsequent weeks (~8 dB/week).
Figure 1:
Noise spectrum and exposure protocol. (A) Spectrum of 16–20 kHz noise presented at 92 dB SPL (analysis bandwidth: 1/15 octave). (B) Noise exposure protocol showing the seven noise exposure levels and recovery period.
2.3. DPOAEs:
Using procedures described previously (Cai et al., 2013; Chen et al., 2010; Sheppard et al., 2015), DPOAEs were measured with a Smart Distortion Product Otoacoustic Emission System (version 4.53, Intelligent Hearing System, Miami, FL). DPOAEs were obtained from the right ear of each animal throughout the study. Measurements were made once before the noise exposure, on the seventh day of each weekly noise exposure, and then one week and two weeks after the last 92 dB SPL noise exposure. Rats were anesthetized with ketamine/xylazine (60 mg/kg/ 6 mg/kg, I.P). Before each DPOAE recording, the external ear canal was examined with an otoscope to exclude the presence of obstructive cerumen, tympanic membrane perforation, or infection. The DPOAE probe assembly with a microphone and two sound delivery tubes coupled to 2 loudspeakers was gently placed in the animal’s external ear canal. DPOAEs were measured with two primary tones (f1 and f2) with an f2/f1 ratio of 1.2. The f1 intensity (L1) was presented 10 dB higher than f2 (L2). L1 was decreased from 80 to 25 dB SPL in 5-dB steps. DPOAE I/O functions for 2f1–f2 SNRs were plotted for f2 frequencies of 4, 6, 8.6, 13.2, 16, 24, and 30 kHz. DPOAE input/output functions were used to define DPOAE thresholds by identifying the intensity at which the DPOAE amplitude was 3 dB above the noise floor along the monotonically increasing input/output function.
For f2 frequencies <20 kHz, the microphone output was sampled at 40 kHz over a period of 204 ms and averaged 32 times and the noise floor was measured in a 24 Hz band surrounding 2f1–f2. For f2 values of ≥20 kHz, the signal was sampled at a rate of 127 kHz over a period of 64 ms, and the noise floor was measured in a 46.7 Hz band surrounding 2f1–f2. During DPOAE testing, body temperature was maintained at 38 °C using a feedback-controlled heating pad. After testing was completed and the animal had recovered from anesthesia; they were returned to their cages in the noise-exposure room in the animal facility.
2.4. Data Analysis:
DPOAE data are presented at f2 frequencies of 6, 8.6, 13.2, 16, 24, and 30 kHz. Statistical analyses were performed using Prism GraphPad v6. DPOAE I/O functions were analyzed for significance using a two-way repeated measures ANOVA analysis and post hoc multiple comparisons performed with Holm-Sidak test.
3. Results
3.1. Effect of Noise on DPOAE Amplitude:
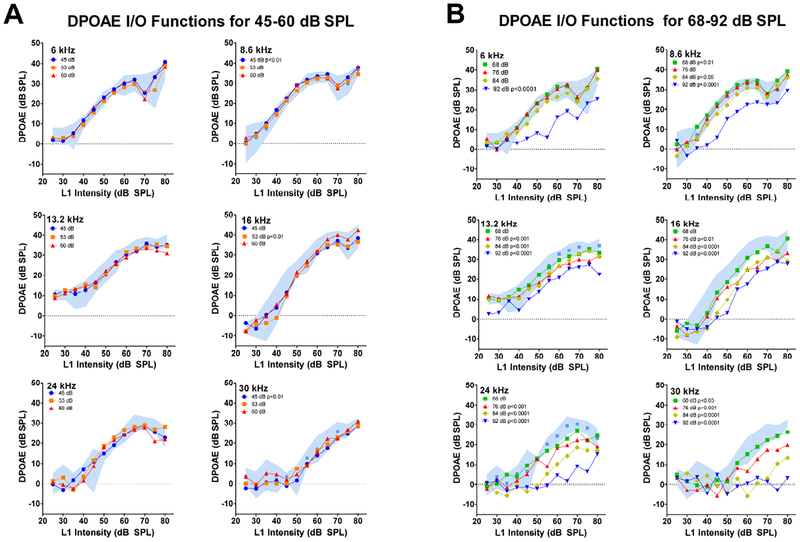
For clarity, the baseline DPOAE are presented 95% confidence intervals around the mean alongside the mean DPOAEs obtained during and after the seven noise exposure intensities. Table 1 shows the frequencies at which DPOAE amplitudes were significantly different from baseline at the indicated noise exposure intensity. Frequencies at which the DPOAE amplitudes were significantly different from baseline are also indicated within each panel of Figure 2 and Figure 4. The I/O functions for the three lowest noise exposures, 45, 53 and 60 dB SPL, are presented in Figure 2A for frequencies of 6, 8.6, 13.2, 16, 24, and 30 kHz. In general, baseline DPOAE amplitudes (blue shaded area, 95% confidence interval) started to increase around 35–40 dB SPL, reaching amplitudes of approximately 30–40 dB SPL at L2 intensities around 70–80 dB SPL. Overall, the mean DPOAE I/O obtained during the 45, 53, and 60 dB SPL noise exposures remained largely within the baseline 95% confidence interval, except in a few cases. After the 45 dB SPL exposure, there was a slight, but significant increase in DPOAE amplitudes at 8.6 kHz (p <0.01) whereas at 30 kHz there was a slight decrease (p <0.01); however, these differences disappeared at the higher exposure levels. Another exception occurred after the 53 dB SPL exposure where a slight decrease was observed at 16 kHz (p <0.01). Thus, the three lowest noise intensities, has relatively little or no effect on DPOAE amplitudes.
Table 1:
Significant changes in DPOAE amplitude. Columns show exposure level (upper) or recovery time (lower), f2 frequency, .F, df, and p values.
| Exposure Level | f2 Frequency | F | df | p |
| 45 dB SPL | 8.6 kHz | 11.02 | 1, 48 | <0.01 |
| 45 dB SPL | 30 kHz | 10.92 | 1, 48 | <0.01 |
| 53 dB SPL | 16 kHz | 12.24 | 1, 48 | <0.01 |
| 68 dB SPL | 8.6 kHz | 10.8 | 1, 48 | <0.01 |
| 68 dB SPL | 30 kHz | 4.93 | 1, 48 | <0.05 |
| 76 dB SPL | 13.2 kHz | 10.02 | 1,48 | <0.01 |
| 76 dB SPL | 16 kHz | 11.64 | 1, 48 | <0.01 |
| 76 dB SPL | 24 kHz | 82.27 | 1, 48 | <0.001 |
| 76 dB SPL | 30 kHz | 17.8 | 1, 48 | <0.001 |
| 84 dB SPL | 8.6 kHz | 4.86 | 1, 48 | <0.05 |
| 84 dB SPL | 13.2 kHz | 16.3 | 1, 48 | <0.001 |
| 84 dB SPL | 16 kHz | 46.9 | 1, 48 | <0.0001 |
| 84 dB SPL | 24 kHz | 82.27 | 1, 48 | <0.0001 |
| 84 dB SPL | 30 kHz | 63.22 | 1, 48 | <0.0001 |
| 92 dB SPL | 6 kHz | 79.23 | 1, 48 | <0.0001 |
| 92 dB SPL | 8.6 kHz | 60.61 | 1, 48 | <0.0001 |
| 92 dB SPL | 13.2 kHz | 45.37 | 1, 48 | <0.0001 |
| 92 dB SPL | 16 kHz | 46.9 | 1, 48 | <0.0001 |
| 92 dB SPL | 24 kHz | 101.2 | 1. 48 | <0.0001 |
| 92 dB SPL | 30 kHz | 139.1 | 1, 48 | <0.0001 |
| Recovery Time | f2 Frequency | F | df | p |
| 1 week Post | 8.6 kHz | 8.23 | 1, 48 | <0.01 |
| 1 week Post | 16 kHz | 10.7 | 1, 48 | <0.01 |
| 1 week Post | 24 kHz | 58.77 | 1, 48 | <0.0001 |
| 1 week Post | 30 kHz | 63.47 | 1, 48 | <0.0001 |
| 2 weeks Post | 16 kHz | 4.19 | 1, 48 | <0.05 |
| 2 weeks Post | 24 kHz | 53.09 | 1, 48 | <0.0001 |
| 2 weeks Post | 30 kHz | 70.75 | 1, 48 | <0.0001 |
Figure 2:
Noise-induced DPOAE input/output functions at f2 frequencies of 6, 8.6, 13.2, 16, 24 and 30 kHz; L1 intensity shown on abscissa. Pre-exposure DPOAE I/O functions shown as 95% confidence interval around the mean (blue shaded area). Mean DPOAE I/O functions obtained during (A) 45, 53 and 60 dB SPL noise exposures and (B) 68, 76, 84 and 92 dB SPL noise exposures. DPOAE input/output functions that were significantly different from pre-exposure input/output function indicated by p value in the legend of each panel (see Table 1 for details).
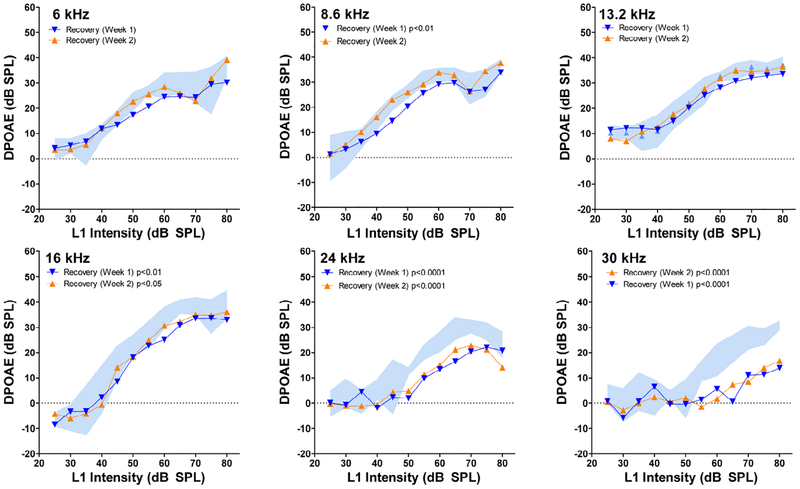
Figure 4:
Post-exposure DPOAE input/output function following the 92 dB SPL noise exposure. DPOAE amplitudes plotted as a function of L1 intensity at f2 frequencies of 6, 8.6, 13.2, 16, 24 and 30 kHz. Blue shaded area represents 95% confidence interval around the mean baseline DPOAEs. Mean post-exposure DPOAE I/O functions obtained 1-week (blue), and 2-weeks (orange) after the 92 dB SPL exposure in the stepwise series of exposues. DPOAE input/output functions that were significantly different from pre-exposure input/output function indicated by p value in the legend of each panel (see Table 1 for details).
Mean DPOAE I/O functions for the four highest noise exposures, 68, 76, 84 and 92 dB SPL are shown in Figure 2B for frequencies of 6, 8.6, 13.2, 16, 24 and 30 kHz. DPOAEs were largely unchanged after the 68 dB SPL exposure, except at two frequencies. There was a slight, but significant increase in DPOAE amplitudes at 8.6 kHz (p <0.01). In contrast, there was a significant decrease in DPOAE amplitudes at 30 kHz (p<0.05). At the three highest intensities, 76, 84 and 92 dB SPL, DPOAE amplitudes generally declined as noise intensity increased; this trend was most evident at high frequencies (24–30 kHz) and less so at low frequencies. Exposure to the 76 dB SPL noise led to a significant decline in DPOAE amplitude at 13.2 kHz (p<0.01), 16 kHz (p<0.01), 24 kHz (p<0.001) and 30 kHz (p<0.001) while lower frequencies remained unchanged. Exposure to the 84 dB SPL noise induced significant DPOAE amplitude changes at all f2 frequencies except 6 kHz. DPOAE amplitudes declined significantly at 8.6 kHz (p<0.05), 13.2 kHz (p<0.001), 16 kHz (p< 0.0001), 24 kHz (p< 0.0001) and 30 kHz (p<0.0001). Finally, the 92 dB SPL exposure caused significant reductions in DPOAE amplitudes at 6 kHz (p<0.0001), 8.6 kHz (p<0.0001), 13.2 kHz (p< 0.0001), 16 kHz (p<0.0001), 24 kHz (p<0.0001), and 30 kHz: (p<0.0001).
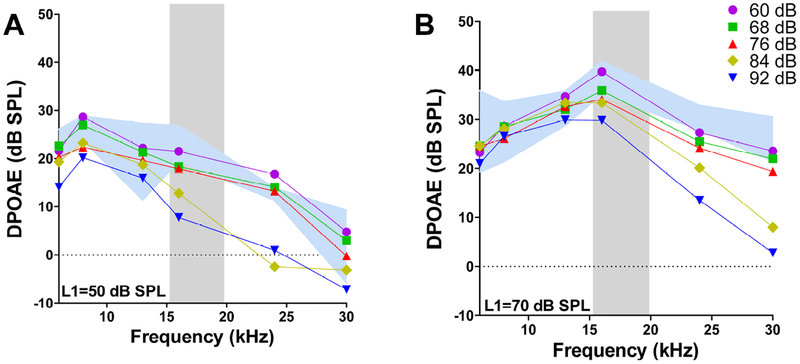
The DPOAE amplitude reductions following the 92 dB SPL exposure occurred over a broad range of primary tone levels and frequencies (Fig. 2B). However, the amplitude reductions at the two lowest frequencies, 6 and 8.6 kHz, mainly occurred at moderate primary tone levels, whereas, the reductions at high frequencies were larger and occurred at all primary tone levels. The smallest amplitude reductions occurred at 13.2 kHz, in the mid-frequency range, and mainly affected DPOAE amplitudes at high primary tone levels. To more clearly illustrate these frequency and level dependent changes, DPOAE amplitudes were plotted as a function of L1 levels of 50 dB SPL (Fig. 3A) and 70 dB SPL (Fig. 3B) for noise exposures ranging from 60 to 92 dB SPL. When L1 was at 50 dB SPL, DPOAE amplitudes were mainly depressed from 16–24 kHz during the 84 dB SPL noise exposure and additionally from 6–8.6 kHz when the exposure level increased to 92 dB SPL. DPOAE amplitudes at 13.2 kHz remained unchanged at all noise exposure levels. When L1 was presented at 70 dB SPL, DPOAE amplitudes were only depressed at frequencies within and above the 16–20 kHz noise band; the magnitude of the decreases increased monotonically with exposure intensity, most notably between 76 and 92 dB SPL.
Figure 3:
Mean DPOAE amplitudes plotted as a function of f2 frequency with the L1 level of f2 set at (A) 50 dB SPL or (B) 70 dB SPL. Data shown for noise exposure levels between 60 and 92 dB SPL. Blue shaded region represents the 95% confidence interval around the mean baseline data. Gray shaded region shows the spectrum of the 10–20 kHz noise exposure.
3.2. DPOAE Recovery:
Figure 4 shows the baseline DPOAE presented as 95% confidence intervals around the mean alongside the mean DPOAEs obtained one and two weeks post-exposure. DPOAE amplitudes were depressed considerably at 30 kHz and to a lesser extent at 24 kHz and there was little evidence of recovery between one and two-weeks post-exposure. At lower frequencies, there was a slight trend of recovery between one and two weeks post-exposure. DPOAE amplitudes were generally within the normal range between 6 and 13.2 kHz at one and two weeks post-exposure. At one week post-exposure, DPOAE amplitudes were significantly different from baseline (Table 1, bottom) at 8.6 kHz (p<0.01), 16 kHz (p<0.01, 24 kHz (p< 0.0001) and 30 kHz (p< 0.0001). At 6 and 8.6 kHz, amplitude reductions occurred mainly at moderate stimulus level, while for 24 and 30 kHz, DPOAEs were reduced at both moderate and high stimulus levels. By two weeks post-exposure, low-frequency DPOAEs were within the normal range. However, DPOAE amplitudes were still significantly below normal at 16 kHz (p<0.05), 24 kHz (p< 0.0001) and 30 kHz (p<0.0001).
3.3. Critical Intensity:
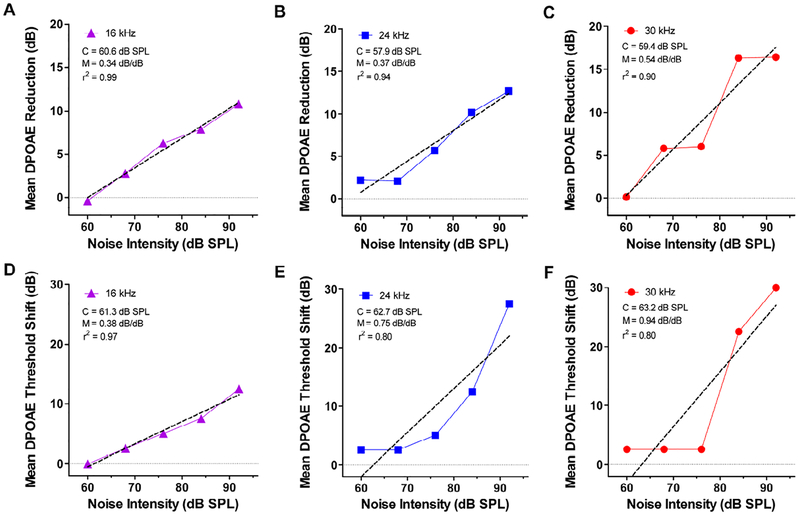
The dose-dependent reductions in DPOAE amplitudes at moderate high stimulus levels can be used to estimate C, the noise intensity above which DPOAE begin to decline. To accomplish this, we measured the mean reduction in DPOAE amplitude between baseline amplitudes and the amplitudes measured after the 60, 68, 76, 84, and 92 dB SPL noise exposure levels. Amplitude reductions were measured at intensities between the baseline DPOAE threshold (45 dB SPL at 30 kHz and 30 dB SPL at 16 and 24 kHz) up to 80 dB SPL. Mean amplitude reduction for 16, 24 and 30 kHz are presented in Figure 5A–C; linear regression was used to fit a line to the data and estimate C, the exposure intensity above which the DPOAEs begin to decline and the slope of the line, M, the rate at which DPOAE amplitude declines with dB increase in exposure intensity. At 16 kHz, C was 60.6 dB SPL (Fig. 5A) and M was 0.34 dB/dB (r2 = 0.987). At 24 kHz, C was 57.9 dB SPL and M was 0.37 dB/dB (r2 = 0.935) (Fig. 5B) while at 30 kHz, C was 59.4 dB SPL and M was 0.54 dB/dB (r2 = 0.90). For frequencies within and above the noise band, C was quite similar, approximately 60 dB SPL; however, the rate at which DPOAE amplitudes declined was frequency dependent; 30 kHz had the steepest slope and 16 kHz the shallowest.
Figure 5:
Mean DPOAE amplitude reductions and mean DPOAE threshold shifts versus noise exposure intensity. Mean reduction in DPOAE amplitude relative to baseline DPOAE amplitudes plotted as function of noise exposure intensities from 60–92 dB SPL for (A) 16 kHz, (B) 24 kHz and (C) 30 kHz. Dashed line shows linear regression fit to the data. The critical intensity (C) and slope (M) and r2 values of the linear regression line are shown in each panel. Mean DPOAE threshold shift relative to baseline thresholds plotted as a function of noise exposure intensities from 60–92 dB SPL for (D) 16 kHz, (E) 24 kHz and (C) 30 kHz. Dashed line shows linear regression fit to the data. Values of C, M and r2 for the linear regression line shown in each panel.
We performed a similar analysis by determining the DPOAE thresholds pre-exposure and DPOAE thresholds during each of the noise exposure. The data were used to determine the DPOAE threshold shift during the 60, 68, 76, 84, and 92 dB SPL noise exposure levels. DPOAE threshold shifts were plotted as a function of noise exposure intensity (Figure 5D–F). Linear regression was used to fit a line to data in order to estimate the critical intensity, C, and the slope, M, i.e., DPOAE threshold shift/dB increase in exposure level above the critical level. The plot of DPOAE threshold shift versus exposure intensity was nearly linear at 16 kHz, but the functions were more curvilinear at 24 kHz and 30 kHz. At 16 kHz (Fig. 5D), C was 61.3 dB SPL and M was 0.38 dB/dB (r2 = 0.97). At 24 kHz, C was 62.7 dB SPL and M was 0.75 dB/dB (r2 = 0.80) (Fig. 5E) while at 30 kHz, C was 63.2 dB SPL and M was 0.94 dB/dB (r2 = 0.80). For test frequencies of 16, 24 and 30 kHz, the values for C were similar, approximately 62 dB SPL. However, the rate at which DPOAE thresholds increased was frequency dependent; 30 kHz had the steepest slope, 0.94 dB thresholds shift per dB exposure intensity; 16 kHz had the shallowest slope. The values of C were a few dB higher for DPOAE thresholds compared to DPOAE amplitude measures, but because the DPOAE amplitudes provided a better fit to the data, further discussion will focus on DPOAE amplitude reductions versus noise exposure level.
4. Discussion
4.1. Critical Level:
There is a growing interest in using prolonged, low-level noise study the neuroplastic changes occurring in the central auditory pathway and possible perceptual changes, some of which could ameliorate tinnitus or hyperacusis (Chen et al., 2014; Formby et al., 2003; Pienkowski et al., 2010; Pienkowski and Eggermont, 2012; Pienkowski et al., 2013; Sheppard et al., 2017; Zheng, 2012). An important prerequisite for interpreting the neurophysiological changes and perceptual alterations is to determine if the functional changes originate in the cochlea or if they are exclusively the result of neural alterations in the central auditory pathway.
Our DPOAE results revealed a significant decline in the 30 kHz DPOAE I/O function at 68 dB SPL (Fig. 3B, 5C) following prolonged exposure to the 16–20 kHz noise. Linear regression analysis (Fig. 5) predicted that the lowest exposure capable of reducing DPOAEs would be slightly above 60 dB SPL. Therefore, our results suggest that noise levels less than 60 dB SPL would be unlikely to impair cochlea function in studies of central auditory plasticity. Increasing the noise level to 76 dB SPL, resulted in substantial reductions in DPOAE amplitudes at f2 frequencies from 16–30 kHz (Fig. 3A–B). The magnitude of the DPOAE reductions were L1-dependent. Noticeable reductions occurred at 16 kHz when L1 was 50 dB SPL (Fig. 3A), whereas little effect was seen at 70 dB SPL (Fig. 3B). These results indicate that it is necessary to assess DPOAEs over a range of frequencies and intensities in order to accurately identify noise-induced DPOAEs changes. One potential limitation of this study is the use of escalating noise levels, which could conceivably “condition” the cochlea, making it more resistant to higher levels noise trauma (Canlon, 1997; Subramaniam et al., 1993). If this were to occur, it would lead to an underestimation of the critical level. Although we observed statistically significant changes and an orderly reduction in DPOAE amplitudes as the exposure intensity increased, the sample size was limited and the study would have benefitted if data were obtained from a larger group of both females and male subjects.
The largest and most consistent noise-induced DPOAE reductions occurred at 24 and 30 kHz, roughly a half to one octave above the noise exposure band, consistent with the well-known noise-induced half-octave or more shifts observed in psychophysical and electrophysiological studies (Carder et al., 1972; Davis et al., 1950; Salvi et al., 1978). At the 92 dB SPL exposure level, DPOAE reductions were also observed one octave below the 16–20 kHz exposure; these reductions were most prominent at moderate L1 levels (Fig. 2). DPOAE results similar to this were also observed in chinchillas at high noise exposure intensities (Eddins et al., 1999).
4.2. Species Differences:
Our linear regression data for inbred Sprague-Dawley rats 16–20 kHz noise can be compared to similar data obtained from chinchillas exposed to octave band noise (2.8–5.6 kHz). The critical level for chinchillas, estimated from DPOAE amplitude reductions, was 50 dB SPL (Eddins et al., 1999); this value was close to the 47 dB SPL estimated from behavioral threshold measures (Carder et al., 1971). The value of C estimated from our Sprague-Dawley DPOAE data was slightly greater than 60 dB SPL, roughly 10 dB higher than that for the chinchilla. Data suggest that the chinchilla is more susceptible to noise-induced hearing loss than humans and other species (DeCory, 1992; Stephenson et al., 1980). Therefore, one possibility is that the difference in C between the chinchilla and inbred Sprague-Dawley rats reflects a species difference. The observed difference in C between Sprague-Dawley rats and chinchillas could also be related to the frequency of the noise exposure. In chinchillas, the value of C was approximately 65 dB SPL for an octave band noise centered at 0.5 kHz versus 47 dB SPL for an octave band centered at 4 kHz, the most sensitive region in the chinchilla audiogram. The 16–20 kHz noise used in our study was also in the most sensitive region of the Sprague-Dawley rat audiogram. Another factor contributing to the differences in C is the bandwidth of the noise, which was a third octave in our study versus an octave wide in studies with the chinchilla. One limitation of our study is that it only assessed male Sprague-Dawley rats; this was done to be consistent with a prior study of ATS using the ABR (Chen et al., 2014). It is conceivable that the critical level and growth of ATS may be influenced by gender and/or the specific strain of inbred animal being evaluated. Therefore, caution should be used when comparing these results to other species, different inbred stains within a species or different genders.
4.3. Assessment Method:
In an earlier study, we used the late peak of the ABR to measure threshold shifts from the same 16–20 kHz noise exposure (Chen et al., 2014). In that study, our regression analysis indicated that C was approximately 77 dB SPL, a value substantially greater than the one we obtained using DPOAEs. This raises the questions as to which metric is more appropriate for estimating the critical level and detecting noise-induced cochlear dysfunction. Based on our previous study of noise-induced hearing loss in chinchillas, we found that C estimated from changes in DPOAEs was nearly identical to that obtained by others using behavioral thresholds (Carder et al., 1972; Eddins et al., 1999). Because these two methods for estimating C were consistent, we suggest that DPOAE amplitude reductions provide a more accurate method of estimating C than ABR thresholds. We also obtained estimates of C using DPOAE threshold shifts; these values were 1–4 dB higher than those obtained using DPOAE amplitude reductions. However, the linear regression fits using thresholds were not as good as those using DPOAE amplitude reductions (Figure 5). These results suggest that DPOAE amplitude reductions provide the best way of estimating the critical level, consistent with our previous results obtained with the chinchilla. Another factor undermining the use of the ABR is that noise-induced hearing loss often leads to enhanced central gain. That is, the later peaks of the ABR are enhanced by cochlear hearing loss (Chambers et al., 2016; Melcher et al., 2009) leading to the underestimation of cochlear pathology. Consistent with this view, we recently reported that prolonged exposure to a 10–20 kHz noise at 75 dB SPL significantly reduced the cochlear compound action potential and summating potential. In contrast, the local field potentials and multiunit spike discharges were enhanced in the inferior colliculus (Sheppard et al., 2017). These results as well as other preliminary results from our lab using a 65 dB SPL exposure suggest that sound levels well below 75 dB SPL can impair cochlear function.
4.4: Recovery:
DPOAE amplitudes at 24 and 30 kHz only partially recovered after the last 92 dB SPL exposure used in this study. Because DPOAEs are generated from the OHC electromotile response, it seems likely that this stepwise series of noise exposures damaged the OHCs in the high frequency region of the cochlea; particularly one-half to an octave above the frequency of the noise exposure (Chen et al., 2014). One explanation for the upward shift in damage is that the traveling wave peak shifts towards the high-frequency base of the cochlea at high sound intensities, a consequence of nonlinear cochlear mechanics (Ramamoorthy et al., 2010; Ruggero and Temchin, 2007). However, another possible explanation for greater loss at the high frequencies is that antioxidant enzyme levels are lower in the base than the apex of the cochlea making the base of the cochlea more vulnerable or fragile to the traumatic effects of noise, aging or ototoxic damage (Sha et al., 2001). The later explanation is appealing because significant reductions in DPOAE amplitudes were observed at the low sound level of 68 dB SPL where nonlinear basilar membrane mechanics would be unlikely to play a major role.
4.4. Summary:
Prolonged exposure to 16–20 kHz noise at 68 dB SPL caused a significant reduction in DPOAE amplitudes at 30 kHz. As the exposure intensity rose from 68 to 92 dB SPL, DPOAE amplitudes decreased primarily between 16 and 30 kHz. Linear regression analysis suggested that the critical intensity at which DPOAE amplitudes begin to decrease occurs at intensities just above 60 dB SPL.
Highlights.
Sprague Dawley rats express a remarkably low critical intensity level.
Prolonged low-level noise exposures cause detrimental cochlear function.
Incrementally enhancing noise can be helpful in distinguishing DPOAE critical intensity levels.
Acknowledgements:
Supported in part by National Institutes of Health grants to AS (F31DC015933) and RS (R01DC014452) and the National Natural Science Foundation of China (Major International Joint Project, No. 81520108015) and the China Scholarship Council (No.201606095027). The funding sources had no role in the design and implementation of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest: None
References
- Barrigón Morillas JM, Gómez Escobar V, Méndez Sierra JA, Vílchez Gómez R, Trujillo Carmona J 2002. An environmental noise study in the city of Cáceres, Spain. Applied Acoustics 63, 1061–1070. [Google Scholar]
- Beierle J 1996. MIR acoustic environment. SAE, Warrendale, PA. [Google Scholar]
- Blakeslee EA, Hynson K, Hamernik RP, Henderson D 1978. Asymptotic threshold shift in chinchillas exposed to impulse noise. J Acoust Soc Am 63, 876–82. [DOI] [PubMed] [Google Scholar]
- Buckey JC Jr., Musiek FE, Kline-Schoder R, Clark JC, Hart S, Havelka J 2001. Hearing loss in space. Aviat Space Environ Med 72, 1121–4. [PubMed] [Google Scholar]
- Cai Q, Whitcomb C, Eggleston J, Sun W, Salvi R, Hu BH 2013. Round window closure affects cochlear responses to suprathreshold stimuli. Laryngoscope 123, E116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canlon B 1997. Protection against noise trauma by sound conditioning. Ear Nose Throat J 76, 248–50, 253–5. [PubMed] [Google Scholar]
- Carder HM, Miller JD 1971. Temporary threshold shifts produced by noise-exposure of long duration. Trans Am Acad Ophthalmol Otolaryngol 75, 1346–54. [PubMed] [Google Scholar]
- Carder HM, Miller JD 1972. Temporary threshold shifts from prolonged exposure to noise. Journal of Speech and Hearing Research 15, 603–23. [DOI] [PubMed] [Google Scholar]
- Chambers AR, Resnik J, Yuan Y, Whitton JP, Edge AS, Liberman MC, Polley DB 2016. Central Gain Restores Auditory Processing following Near-Complete Cochlear Denervation. Neuron 89, 867–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Decker B, Krishnan Muthaiah VP, Sheppard A, Salvi R 2014. Prolonged noise exposure-induced auditory threshold shifts in rats. Hear Res 317, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Kermany MH, D’Elia A, Ralli M, Tanaka C, Bielefeld EC, Ding D, Henderson D, Salvi R 2010. Too much of a good thing: long-term treatment with salicylate strengthens outer hair cell function but impairs auditory neural activity. Hear Res 265, 63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark WW 1991. Recent studies of temporary threshold shift (TTS) and permanent threshold shift (PTS) in animals. J Acoust Soc Am 90, 155–63. [DOI] [PubMed] [Google Scholar]
- Davis H, Morgan CT, Hawkins JE, Galambos R, Smith FW 1950. Temporary deafness following exposures to loud tones and noise. Acta Otolaryngologica (Stockholm) Supp. 88, 1–57. [PubMed] [Google Scholar]
- DeCory L, Dancer AL, Aran JM 1992. Species differences and mechanisms of damage In: Dancer AL, Henderson D, Salvi R, Hamernik RP (Eds), Noise-Induced Hearing Loss. St. Louis, MO: Mosby Year Book, 73–88. [Google Scholar]
- Eddins AC, Zuskov M, Salvi RJ 1999. Changes in distortion product otoacoustic emissions during prolonged noise exposure. Hear Res 127, 119–28. [DOI] [PubMed] [Google Scholar]
- Formby C, Sherlock LP, Gold SL 2003. Adaptive plasticity of loudness induced by chronic attenuation and enhancement of the acoustic background. J Acoust Soc Am 114, 55–8. [DOI] [PubMed] [Google Scholar]
- Henry JA, Schechter MA, Zaugg TL, Griest S, Jastreboff PJ, Vernon JA, Kaelin C, Meikle MB, Lyons KS, Stewart BJ 2006. Clinical trial to compare tinnitus masking and tinnitus retraining therapy. Acta Otolaryngol Suppl, 64–9. [DOI] [PubMed] [Google Scholar]
- Kheirbek I, Ito K, Neitzel R, Kim J, Johnson S, Ross Z, Eisl H, Matte T 2014. Spatial variation in environmental noise and air pollution in New York City. Journal of Urban Health 91, 415–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, Zhang JW, McPherson B, Pienkowski M, Wu EX 2015. Long-term, passive exposure to non-traumatic acoustic noise induces neural adaptation in the adult rat medial geniculate body and auditory cortex. Neuroimage 107, 1–9. [DOI] [PubMed] [Google Scholar]
- Melcher JR, Levine RA, Bergevin C, Norris B 2009. The auditory midbrain of people with tinnitus: abnormal sound-evoked activity revisited. Hear Res 257, 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick W 1991. Human temporary threshold shift (TTS) and damage risk. J Acoust Soc Am 90, 147–54. [DOI] [PubMed] [Google Scholar]
- Mills JH 1973. Temporary and permanent threshold shifts produced by nine-day exposures to noise. Journal of Speech and Hearing Research 16, 426–38. [DOI] [PubMed] [Google Scholar]
- Mills JH, Talo SA 1972. Temporary threshold shifts produced by exposure to high-frequency noise. Journal of Speech and Hearing Research 15, 624–31. [DOI] [PubMed] [Google Scholar]
- Mills JH, Adkins WY, Gilbert RM 1978. High-frequency hearing losses caused by low-frequency noises. Otolaryngology 86, ORL-821–3. [DOI] [PubMed] [Google Scholar]
- Mills JH, Gilbert RM, Adkins WY 1979. Temporary threshold shifts in humans exposed to octave bands of noise for 16 to 24 hours. J Acoust Soc Am 65, 1238–48. [DOI] [PubMed] [Google Scholar]
- Norena AJ, Chery-Croze S 2007. Enriched acoustic environment rescales auditory sensitivity. Neuroreport 18, 1251–5. [DOI] [PubMed] [Google Scholar]
- Pienkowski M, Eggermont JJ 2009. Long-term, partially-reversible reorganization of frequency tuning in mature cat primary auditory cortex can be induced by passive exposure to moderate-level sounds. Hear Res 257, 24–40. [DOI] [PubMed] [Google Scholar]
- Pienkowski M, Eggermont JJ 2010. Passive exposure of adult cats to moderate-level tone pip ensembles differentially decreases AI and AII responsiveness in the exposure frequency range. Hear Res 268, 151–62. [DOI] [PubMed] [Google Scholar]
- Pienkowski M, Eggermont JJ 2012. Reversible long-term changes in auditory processing in mature auditory cortex in the absence of hearing loss induced by passive, moderate-level sound exposure. Ear Hear 33, 305–14. [DOI] [PubMed] [Google Scholar]
- Pienkowski M, Munguia R, Eggermont JJ 2013. Effects of passive, moderate-level sound exposure on the mature auditory cortex: spectral edges, spectrotemporal density, and real-world noise. Hear Res 296, 121–30. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Zha DJ, Nuttall AL 2010. The biophysical origin of traveling-wave dispersion in the cochlea. Biophys J 99, 1687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero MA, Temchin AN 2007. Similarity of traveling-wave delays in the hearing organs of humans and other tetrapods. J Assoc Res Otolaryngol 8, 153–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi RJ, Hamernik RP, Henderson D 1978. Discharge patterns in the cochlear nucleus of the chinchilla following noise induced asymptotic threshold shift. Experimental Brain Research 32, 301–320. [DOI] [PubMed] [Google Scholar]
- Saunders JC, Mills JH, Miller JD 1977. Threshold shift in the chinchilla from daily exposure to noise for six hours. J Acoust Soc Am 61, 558–70. [DOI] [PubMed] [Google Scholar]
- Saunders JC, Cohen YE, Szymko YM 1991. The structural and functional consequences of acoustic injury in the cochlea and peripheral auditory system: a five year update. J Acoust Soc Am 90, 136–46. [DOI] [PubMed] [Google Scholar]
- Sha SH, Taylor R, Forge A, Schacht J 2001. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear. Res 155, 1–8. [DOI] [PubMed] [Google Scholar]
- Sheppard AM, Chen GD, Salvi R 2015. Potassium ion channel openers, Maxipost and Retigabine, protect against peripheral salicylate ototoxicity in rats. Hear Res 327, 1–8. [DOI] [PubMed] [Google Scholar]
- Sheppard AM, Chen GD, Manohar S, Ding D, Hu BH, Sun W, Zhao J, Salvi R 2017. Prolonged low-level noise-induced plasticity in the peripheral and central auditory system of rats. Neuroscience 359, 159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson MR, Nixon CW, Johnson DL 1980. Identification of the minimum noise level capable of producing an asymptotic temporary threshold shift. Aviat Space Environ Med 51, 391–6. [PubMed] [Google Scholar]
- Subramaniam M, Henderson D, Spongr V 1993. Effect of low-frequency “conditioning” on hearing loss from high-frequency exposure. Journal of the Acoustical Society of America 93, 952–6. [DOI] [PubMed] [Google Scholar]
- Syka J, Popelar J 1980. Hearing threshold shifts from prolonged exposure to noise in guinea pigs. Hear Res 3, 205–13. [DOI] [PubMed] [Google Scholar]
- Wang J, Powers NL, Hofstetter P, Trautwein P, Ding D, Salvi R 1997. Effects of selective inner hair cell loss on auditory nerve fiber threshold, tuning and spontaneous and driven discharge rate. Hear Res 107, 67–82. [DOI] [PubMed] [Google Scholar]
- Zheng W 2012. Auditory map reorganization and pitch discrimination in adult rats chronically exposed to low-level ambient noise. Front Syst Neurosci 6, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Merzenich MM 2012. Environmental noise exposure degrades normal listening processes. Nat Commun 3, 843. [DOI] [PubMed] [Google Scholar]