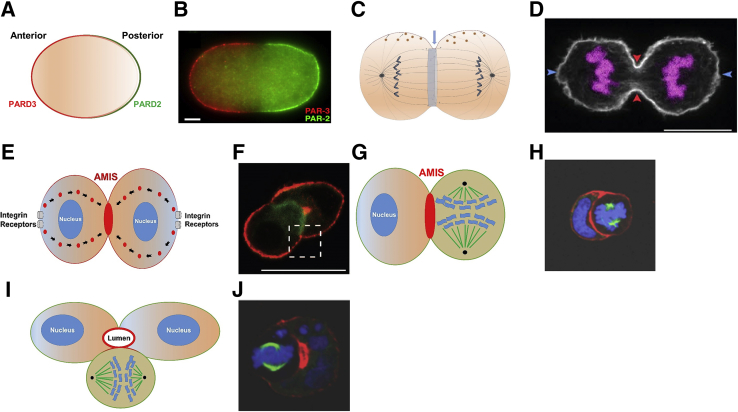
Figure 2.
Transition to multicellularity. A and B: In the one-cell zygote, fertilization triggered asymmetric redistribution of anterior [partitioning defective (PARD3; alias PAR-3)] and posterior (PARD2; alias PAR-2) polarity determinants within the cortex.34A: Schematic showing cortical localization of anterior (PARD3) and posterior (PARD2) polarity determinants. B:Caenorhabditis elegans zygote, stained for PAR-3 (PARD3; red) and PAR-2 (PARD2; green). Other polarity determinants are not shown. C and D: Conserved polarization processes enabled bipolar mitotic spindle assembly, positioning of the cleavage furrow, and cell membrane expansion in epithelial cells. C: Schematic shows that chromosomal DNA is linked by microtubules to spindle pole centrosomes that are anchored to the cell cortex by astral microtubules.36 Motor proteins drive equal genome segregation.37 Via microtubules, the spindle directed transport of lipid-containing vesicles (orange circles) to membrane growth regions.38 The actomyosin ring (light blue) provided the contractile force for cell cleavage39 (blue arrow), set perpendicular to the spindle plane.6D: Advanced cytokinesis in an epithelial cell. Blue and red arrows indicate spindle poles and the cleavage furrows, respectively. Chromosomal DNA (purple). E and F: After completion of cytokinesis, the new cell doublet engaged extracellular matrix (ECM) via cell membrane integrin receptors. This process promoted transcytosis of membrane components from the basal domain to the cell–cell contact region that becomes the apical membrane initiation site (AMIS). E: Schematic shows integrin/ECM-mediated trancytosis of membrane components with apical characteristics (red circles) from basolateral domains to the AMIS (red oval). F: Early epithelial cell doublet stained for Podxl (apical marker) and β-integrin green fluorescent protein (GFP). Podxl was expressed at the ECM-facing basolateral membrane and underwent directed vesicular transport to the nascent AMIS. Boxed area was selected for high power (HP) magnification in original study (not shown).9G and H: From the two-cell stage, the mitotic spindle controlled the alignment of apical membrane components. G: Schematic shows developing AMIS at contact site between resting and dividing cells of the doublet. H: Caco-2 doublet containing resting and dividing cells stained for DNA (blue), tubulin (green), and filamentous actin (red). I and J: In subsequent divisions, the spindle was oriented to maintain apical membrane position in the center of developing glands, surrounded by an epithelial monolayer. I: Schematic showing orientation of a cell monolayer around central lumen. J: Developing Caco-2 glandular structure (gland) containing resting cells and one dividing cell, stained for DNA (blue), tubulin (green), and filamentous actin (red). Scale bars: 5 μm (B); 10 μm (D); 20 μm (F).
Reprinted from Nance and Zallen35 with permission from Development (B); from Spira et al36 with permission from eLife (D); from Bryant et al9 with permission from Elsevier (F); and from Jaffe et al6 with permission from the Journal of Cell Biology (H and J).