Abstract
Purpose
To analyse the impact of female characteristics on assisted reproductive technology outcome among male haematological cancer survivors.
Methods
A retrospective analysis of 93 haematological cancer survivors attending our tertiary referral fertility centre between June 1998 and June 2017 for achieving fatherhood with assisted reproductive technology treatments.
Results
A progressive increase in the median female age was observed during the study period (32.2 years until the year 2007 and 36.9 years from the year 2012). Fifty-five out of 93 patients were treated with intracytoplasmic sperm injection (ICSI) (113 ovarian stimulations, 108 ICSI procedures). Cryopreserved ejaculated sperm was used in 28 couples, fresh sperm in 19, and thawed testicular sperm in 8 couples. Mean female age at ovarian stimulation was 37.0 ± 4.7 years. Twenty-six pregnancies resulted in a full-term birth (23% per started ovarian stimulation; 43.6% per couple) and 33 children were born. No significant differences were observed according to source of sperm (fresh, frozen, testicular) and multivariate analysis confirmed that maternal age was the only variable inversely related to the cumulative delivery rate, being five times lower (15.7%) when the female partner was ≥ 40 years (OR = 0.22, 95% CI 0.06–0.77) vs. 58.3% with younger women (p = 0.0037).
Conclusions
Delayed childbearing and female ageing affect ICSI outcome in couples where the male is a survivor of haematological cancer. This topic should be discussed when counselling male cancer patients about fertility preservation.
Keywords: Assisted reproductive technology, Haematological cancer, Male infertility, Delayed childbearing, Sperm banks
Introduction
Antineoplastic treatments for lymphatic or haematological malignancies impair spermatogenesis with a high risk of transient or irreversible infertility. An unfavourable outcome depends on several factors, as quality of spermatogenesis before treatment, type of malignancy, cancer treatment protocol and patient susceptibility [1, 2]. Nevertheless, it is impossible to predict whether a patient will become azoospermic or whether any of the sperm parameters will be impaired after the completion of antineoplastic therapy. Since fertility is an important factor for the quality of life of male patients with a history of haematological malignancies, to improve their chance of having biological children in the future, sperm should be cryopreserved [3, 4]. However, sperm cryopreservation is not always feasible like in pre-pubertal boys (< 11 years old) [5], or for patients unable to provide sperm by masturbation, or for those needing immediate chemotherapy. When oncologic therapy causes irreversible azoospermia, testicular sperm extraction (TESE) can be still attempted [6].
Assisted reproductive technologies (ARTs) have recently improved the chance of parenthood, even for patients with severe sperm defects [7]. Cancer survivors of lymphatic or haematological tumours have the highest rate of ART usage [8, 9] because bone marrow transplantation frequently causes irreversible testicular damage (38% in our population). This treatment increases the risk of irreversible testicular damage and decreases the probability of resumption of spermatogenesis.
In a recent systematic review of the literature referring to unselected cancer survivors [10], ART was reported to give high chances of pregnancy (30%) and of live birth per cycle (25%). Cumulatively, 49% of cancer survivors had at least one child using ART or intrauterine insemination with cryopreserved semen. As these data refer to papers published for the most part before 2010 and the female age is not mentioned, we have retrospectively analysed a group of infertile haematological cancer survivors, to understand whether female ageing affects ART outcomes in these selected groups of cancer patients. Furthermore, we have compared our data with those of similar case studies for male lymphatic or haematological malignancies disease.
Materials and methods
Selection of participants and data collection
Male patients referred to our fertility centre with a diagnosis of “haematological malignancy” and that cryobanked sperm were selected by a query in our electronic clinical database. The information extracted from the medical records included age, partner age, cancer type, date of cryopreservation, oncologic follow-up, semen analyses after treatments, interest in reproduction, and spontaneous pregnancies achieved before or after cancer treatment. ART procedures performed during the 20-year period were also analysed. This retrospective study was approved by our hospital’s institutional ethics committee and all patients provided written informed consent for the scientific use of their clinical data.
Sperm banking and testicular sperm extraction
Patients who cryopreserved semen signed an informed consent providing the medical indications and the terms and conditions for the cryopreservation of their sperm. Semen samples were analysed according to World Health Organisation (WHO) criteria [11–13], then loaded in straws (Cryo Bio System), and cryopreserved following a rapid two-phase protocol [14]. Diagnosis of azoospermia was given after centrifugation of the entire semen sample. Patients were contacted yearly by trained health personnel about their intentions to keep their semen samples stored. When patients were ready to attempt reproduction male and female counselling was offered in our centre. For patients with irreversible azoospermia who did not cryopreserve semen, testicular sperm extraction (TESE) was offered. The procedure was performed as day surgery using open biopsy on both testes [15]. To estimate the amount of retrieved spermatozoa, a five-degree scale as described by Hauser et al. [16] was adopted. TESE was considered successful when sample was ≥ 2 degrees (at least a single spermatozoon observed in each microscopic field) and suitable for at least one intracytoplasmic sperm injection (ICSI) cycle with thawed sperm.
For patients with unsatisfactory fertility recovery, or when a female factor could have reduced the chances of pregnancy, ICSI [7] was offered.
Female population
Ovarian reserve testing included cycle day 3 estradiol and serum follicle-stimulating hormone (FSH) levels until 2010, while antral follicle count (AFC) [17] and serum AMH levels [18] were added in the evaluation thereafter. Female infertility co-factor aetiologies were grouped into low ovarian reserve [19], endometriosis, polycystic ovarian syndrome, and tubal factor. ICSI outcomes in oncologic patients were compared with the results of 132 non-oncologic couples (controls) of the same age during the same treatment period.
Intracytoplasmic sperm injection
The protocols for ovarian stimulation consisted of GnRH agonist long protocol, GnRH antagonist protocol, or flare-up GnRH agonist protocol and recombinant follicle-stimulating hormone (rFSH) or human menopausal gonadotrophins (HMG) with starting doses determined according to anti-Müllerian hormone (AMH), antral follicle count (AFC), and body mass index (BMI) or considering previous treatment cycles in patients at repetitive attempts.
Long GnRH agonist protocol was based on the administration of daily leuprorelin (Enantone die, Takeda, Italy) or triptorelin depot (3.75 mg IM, Decapeptyl®, Ipsen, Milan, Italy) on day 21 of the previous luteal phase of the stimulation cycle. When pituitary desensitisation was achieved (14 days after the initiation of GnRH agonist), as evidenced by the absence of ovarian follicles > 10 mm and endometrial thickness < 5.4 mm on transvaginal ultrasound examination, gonadotropin stimulation was initiated. In the GnRH antagonist protocol, women received a low-dose oral contraceptive started on cycle day 1 of the previous cycle for 21 days. From 3 to 4 days after the last pill, the first day of withdrawal bleeding gonadotropin stimulation was initiated and when the leading follicle reached 13–14 mm in mean diameter, and/or plasma E2 exceeded 400 pg/ml, an injection of 0.25 mg of GnRH antagonist (Cetrotide®, Merck Serono, Rome, Italy; Orgalutran®, Organon, MSD-Italy) was administered SC daily until the day of ovulation trigger.
In the flare-up GnRH protocol, women on day 1 of their spontaneous menstrual or a withdrawal bleeding after receiving a low-dose oral contraceptive started on cycle day 1 of the previous cycle for 15–21 days, triptorelin (0.025 mg/day) and gonadotropins were started.
A starting variable dose of gonadotropin rFSH (Puregon®, MSD-Italy; Gonal-F, Serono, Rome, Italy) or hMG (Meropur®, Ferring, Milan, Italy) for the first 4 days and then an individualised dose was administered according to the parameters resulting from transvaginal ultrasound and estradiol and progesterone levels until the day of ovulation trigger. When at least three follicles with a mean diameter > 18 mm were observed, 250 μg of recombinant human chorionic gonadotropin (hCG) (Ovitrelle; Merck Serono) was administered subcutaneously. Transvaginal oocyte retrieval was performed 36 h after hCG injection. Embryo transfer was performed 48–120 h after oocyte collection. Luteal phase was supported in all patients with vaginal progesterone (Crinone 8%; Merck Serono or Prometrium; Rottapharm). Serum hCG was assessed 2 weeks after embryo transfer and then every 48 h until a value over 1.000 mIU was detected and a vaginal ultrasound was scheduled 4 weeks after the embryo transfer.
ICSI procedure was performed as previously described [7]. After injection, oocytes were incubated in 20 μL drops at 37 °C, 5% O2 and 5% CO2; fertilisation was assessed after 17–20 h and embryo cleavage 24 h thereafter. Embryos were transferred into the uterine cavity 48–72 or 120 h (day 2–day 3 or day 5) after ICSI. All patients were given the option of oocyte freezing (since during the period 2004–2009 only oocyte cryopreservation was allowed) or embryos [20]. Clinical pregnancy was defined as the ultrasound visualisation of one or more gestational sacs. A live birth event means either a single or a multiple birth.
Data analysis
Data were described as numbers and percentages, or means and standard deviations, as appropriate. Differences among groups were explored with the Kruskal-Wallis test or chi-square test with Fisher correction, as appropriate. Possible risk factors for pregnancy were analysed with logistic regression analysis. All factors with a p value less than 0.1 were subjected to stepwise multivariable analysis. A p less than 0.05 was considered as significant. All analyses were performed using Stata13 (2013, Stata Corp, TX, USA, www.stata.com).
Results
A total of 213 patients with a diagnosis of haematological malignancy attended our fertility centre between June 1998 and June 2017 (Table 1). The age at oncologic treatment start was 28.6 ± 9.5 (SD) years (range 2.6–49.5 years). The cancer diagnoses were Hodgkin’s lymphoma (n = 110), non-Hodgkin’s lymphoma (n = 69), leukaemia (n = 29), and myelomas (n = 5) (Table 1). Of patients who were older than 30 years old at treatment start (n = 97; median age 35.8 years, range 30.3–49.5 years), 12 had completed their family (12.3%), a lower value compared to other studies [9, 21, 22].
Table 1.
Demographic characteristics of male cancer survivor patients
| No. | |
|---|---|
| Patients (no. and age at diagnosis) | 213 (28.6 ± 9.5) |
| Hodgkin’s lymphoma | 110 (27.4 ± 8.2) |
| Non-Hodgkin’s lymphoma | 69 (32.2 ± 7.8) |
| Leukaemia | 29 (22.7 ± 13.6) |
| Myelomas | 5 (39.1 ± 2.6) |
| Cryopreservation | 174 (9 azoospermic) |
| No cryopreservation | 39 (25 azoospermic) |
| Testicular sperm extraction (24/34 azoospermic) | 10 positive (41.6%) |
| Patients with procreative motivations (mean age) | 93 (37.7 ± 6.2) |
| Interval from cancer diagnosis (mean years ± SD) | 10.5 ± 8.7 (range 0.2–33.1) |
| Cryopreservation | 57 (5 azoospermic) |
| No cryopreservation | 36 (19 azoospermic) |
| Testicular sperm extraction (24 patients) | 10 positive (41.6%) |
| Female age (mean years ± SD) | 35.4 ± 5.1 |
| Spontaneous pregnancies | 11 |
| Couples undergoing ICSI | 55 |
| Other plans | 27 |
Of the 156 patients who attempted sperm cryopreservation before oncologic treatments, 148 were successful with a mean of 13 cryopreserved vials (range 1–57). Eight patients were found to be azoospermic prior to cancer treatment. Additionally, 18 patients cryopreserved semen after at least one chemotherapy cycle (mean of 8 vials, range 0–25), but one was found to be azoospermic. The remaining 39 patients did not cryopreserve sperm before antineoplastic therapy. Of these, 14 were subfertile and 25 azoospermic. Failure to bank sperm was due to their young age at diagnosis (8 patients < 13 years), refusal justified by paternity prior to diagnosis (5 patients), or lack of information (26 patients).
A total of 24 azoospermic patients (5 at the time of sperm banking, 19 who had not cryopreserved) underwent TESE. Spermatozoa were recovered and frozen in 10 patients (range 1–8 cryopreserved vials). Fourteen patients had complete spermatogenic failure (Sertoli cell-only syndrome). No difference in FSH values was present in the two groups: 15.8 ± 6.2 (SD), range 2.15–28.8 mU/ml vs. 22.8 ± 10.8 (SD), range 7.7–45 mU/ml (p, ns). Some of the azoospermic men were offered adoption or donor sperm (Table 1).
Patients with procreative motivations
During the analysed period, 93 patients tried to reproduce. The average age of the patients was 37.7 ± 6.2 (SD) years (range 26.2–61.9 years). The mean time interval from the cancer diagnosis was 10.5 ± 8.7 years (range 0.2–33.1 years). A long wait time (> 15 years) was often related to the onset of the cancer at a young age (18/27 were under 20 years old). Clinical and reproductive characteristics of male cancer survivors with procreative motivations are reported in Table 2.
Table 2.
Clinical and reproductive characteristics of male cancer survivors with procreative motivations
| Cases | SCT | CT | CT + RT | RT | Unknown | Natural pregnancy | ICSI pregnancy | TESE failure | |
|---|---|---|---|---|---|---|---|---|---|
| 44 | Hodgkin’s lymphoma | 14 | 7 | 17 | 2 | 4 | 5 | 13 | 6 |
| 28 | Non-Hodgkin’s lymphoma | 11 | 8 | 7 | 1 | 1 | 6 | 3 | 5 |
| 19 | Leukaemia | 9 | 6 | 4 | 0 | 0 | 0 | 8 | 2 |
| 2 | Myeloma | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 93 | Patients | 36 | 21 | 28 | 3 | 5 | 11 | 24 | 14 |
| 11 | Natural pregnancy | 1 | 2 | 8 | 0 | 0 | |||
| 24 | ICSI pregnancy | 7 | 8 | 7 | 1 | 1 | |||
| 32 | ICSI failure | 17 | 6 | 4 | 1 | 4 | |||
| 14 | TESE failure | 8 | 2 | 3 | 1 | 0 | |||
| 5 | Looking for children | 0 | 1 | 4 | 0 | 0 | |||
| 6 | No | ICSI | 3 | 1 | 2 | 0 | 0 | ||
| 2 | Died | 1 | 1 | 0 | 0 | 0 |
Cumulative pregnancy rate (spontaneous + IVF) in Hodgkin’s lymphoma (40.9%) does not differ from non-Hodgkin’s lymphoma (32.1%) and leukaemia (42.1%). Noteworthy is the low spontaneous pregnancy rate in leukaemia’s (0/19) and after stem cell transplantation (1/36)
SCT stem cell transplant, CT chemotherapy, RT radiotherapy, unknown data not available for therapies performed many years earlier in other hospitals, TESE testicular sperm extraction
Fourteen azoospermic patients (15%) were unable to proceed due to lack of sperm during TESE. Of these patients, 4 had attempted sperm freezing but were already azoospermic, while the remaining 10 did not cryopreserve sperm before oncologic treatments. Two of these couples resorted to IVF with donor sperm. One couple achieved a full-term pregnancy (wife aged 33.5 years), while the other did not get pregnant (wife aged 41.8 years).
Two patients who successfully cryopreserved ejaculated (n = 1) or testicular sperm (n = 1) died prior to IVF due to recurrence of Hodgkin’s and non-Hodgkin’s lymphomas.
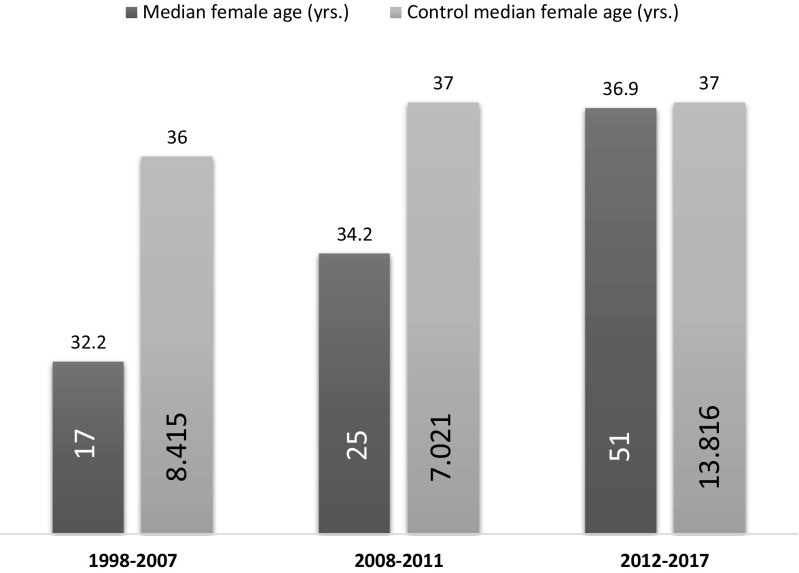
Six have not proceeded with ICSI, while 5 men with normal semen parameters have just started trying to reproduce. Finally, 11 patients (11.8%) achieved a full-term spontaneous pregnancy (15 healthy children born), 5.9 ± 2.8 (SD) years after the diagnosis (range 1.7–10.7 years). Median female age of the latter group was 33 years. The mean female age of these 93 couples was 35.4 ± 5.1 (range 24.6–47.5 years), and as shown in Fig. 1, we observed a progressive increase in the women’s mean age over the two decades examined (Fig. 1). Until the year 2007, 17 couples had requested reproductive assistance with a median female age of 32.2 years (p = 0.009 vs. control). In the period 2008–2011, the median age was 34.2 years (p = 0.057 vs. control), while during 2012–2017, it had risen to 36.9 years. (p = 0.19 vs. control).
Fig. 1.
Age of the female partner at the start of infertility treatment. Female age has been stable over the last 20 years in the general population (light grey bar), while the study population (dark grey bar) has shown a trend towards an increase (p = 0.069), reaching the level of the general population in the last years
Overall, 55 couples entered an ART programme with controlled ovarian stimulation (COS) (in total 113 COS cycles). Female age at COS was 37.0 ± 4.7 years. Sixteen women had a reduced ovarian reserve at COS (29.1%) and 3 had ovarian endometriosis (5.4%). ICSI was routinely used. Five ICSI were cancelled due to poor ovarian response (n = 4) or absence of testicular spermatozoa after thawing (n = 1). Of the remaining 108 COS attempts with ICSI, cryopreserved sperm was used in 52 cycles, fresh sperm in 35, and, finally, testicular in 21. No differences in fertilisation rate (p = 0.417), implantation rate (p = 0.357), pregnancy (p = 0.145), and delivery rate (p = 0.322) were observed according to sperm source, albeit higher fertilisation failure rate was observed for testicular sperm (5 out 21, 23.8%) (p = 0.052). In 5 couples, the embryo transfer was postponed due to the risk of ovarian hyperstimulation syndrome. In total, 195 fresh and 42 cryopreserved embryos were transferred in 93 and 22 procedures, respectively. Twenty-six pregnancies resulted in a full-term birth (23% per started controlled ovarian stimulation; 43.6% per couple) and overall 33 children were born (Table 3). The cumulative full-term birth rate per started controlled ovarian stimulation was similar in both study population and control population (23% vs. 21.6%) (p = 0.878).
Table 3.
ICSI characteristics and outcomes
| Total | Banked sperm | Fresh sperm | Testicular sperm | p | Controls | p total vs. control | |
|---|---|---|---|---|---|---|---|
| Couples | 55 | 28 | 19 | 8 | 132 | ||
| Started cycles (COS) | 113 | 56 | 35 | 22 | 134 | ||
| Suspended cycles | 5 (4.4%) | 4 (7.1%) | 0 | 1 (4.6%) | 0.335 | 4 (3.0%) | 0.736 |
| Female age at COS | 37.0 ± 4.7 | 37.2 ± 4.8 | 36.4 ± 4.3 | 37.3 ± 4.8 | 0.593 | 37.0 ± 4.3 | 0.978 |
| Cycles with oocyte retrieval | 108 (95.6%) | 52 (92.9%) | 35 (100%) | 21 (95.5%) | 0.335 | 130 (97.0) | 0.736 |
| Retrieved oocytes | 9.5 ± 6.7 | 9.3 ± 7.4 | 9.7 ± 6.2 | 10.0 ± 5.8 | 0.583 | 10.6 ± 6.7 | 0.180 |
| Injected oocytes | 5.6 ± 4.5 | 5.8 ± 5.5 | 5.0 ± 2.5 | 6.0 ± 4.3 | 0.733 | 6.0 ± 3.3 | 0.045 |
| Fertilised oocytes | 3.6 ± 3.4 | 3.7 ± 4.4 | 3.4 ± 1.9 | 3.7 ± 2.8 | 0.675 | 4.4 ± 2.7 | 0.003 |
| Fertilisation rate | 65.1 ± 28.6% | 64.7 ± 31.9% | 70.0 ± 22.5% | 58.0 ± 28.8% | 0.417 | 73.0 ± 25.7% | 0.025 |
| Fertilisation failure | 10 (9.3%) | 4 (7.7%) | 1 (2.9%) | 5 (23.8%) | 0.052 | 6 (4.6%) | 0.196 |
| No. of fresh/cryo ET | 93/22 | 47/12 | 32/5 | 14/5 | 117/45 | ||
| Fresh embryos transferred | 2.1 ± .0.7 | 2.1 ± 0.8 | 2.1 ± 0.6 | 2.1 ± 0.5 | 0.279 | 2.1 ± 0.7 | 0.512 |
| Cryo embryos transferred | 1.9 ± 1.0 | 2.1 ± 1.2 | 1.6 ± 0.5 | 1.8 ± 0.8 | 0.898 | 1.3 ± 0.5 | 0.016 |
| Cumulative IR (%) | 18.2 ± 34.2 | 13.1 ± 31.1 | 22.5 ± 34.5 | 25.4 ± 41.7 | 0.357 | 17.6 ± 33.3 | 0.896 |
| Cumulative PR (%) | 30 (26.1%) | 11 (19.6%) | 13 (37.1%) | 6 (27.2%) | 0.145 | 41 (25.3%) | 0.890 |
| Miscarriages (%) | 4/30 (13.3%) | 1/11 (9.1%) | 2/13 (15.4%) | 1/6 (16.6%) | 1.000 | 9/41 (22.0%) | 0.536 |
| Deliveries per COS | 26 (23.0%) | 10 (17.9%) | 11 (31.4%) | 5 (22.7%) | 0.322 | 29 (21.6%) | 0.878 |
| Deliveries per couple | 24 (43.6%) | 9 (32.1%) | 10 (52.6%) | 5 (62.5%) | 0.220 | 29 (22.0%) | 0.004 |
| Born children | 33 | 12 | 15 | 6 | 40 | ||
| Preterm birth (no. of child) | 10 | 2 | 6 | 2 | 7 | ||
| Low birth weight (no. of child) | 11 | 2 | 7 | 2 | 10 |
Values are mean ± SD or number (percentage). COS controlled ovarian stimulation, ET embryo transfer, IR implantation rate, PR pregnancy rate
No major malformations were reported during the paediatric follow-up. The proportion of live-born males out of all live births was 0.45 (15/33). Preterm births (22–36 completed weeks of gestation) resulted from 5/7 twin pregnancies (range 27–35 weeks) and low birth weights (500–2499 g) were observed in 11 newborns (range 0.57–2.4 kg), 10 twins and 1 singleton. In the control population, preterm births and the number of low birth weights were not significantly different (Table 3).
The prognostic factors for successful ICSI outcome were evaluated by univariate logistic regression analysis. Factors with a p value < 0.1 were submitted to multivariate logistic regression analysis (Table 4). Although fresh sperm was more effective than banked sperm and testicular sperm, multivariate analysis established that only the maternal age was inversely related to the risk of full-term pregnancy. When the female partner was ≥ 40 years at COS, the probability of live birth was five times lower (OR = 0.22, 95% CI 0.06–0.77). In detail, 3 out of 19 couples had a child when the female partner was ≥ 40 years old at COS (15.7% cumulative delivery rate/couple) vs. 21 out of 36 in younger women (58.3%/couple; p = 0.0037). Two young couples had second pregnancy (23 full-term pregnancies out of 36 couples). In couples achieving pregnancy after ART, the mean female age was 33.9 years (range 25.4–41), significantly lower than that of couples not achieving pregnancy (37.3 years; range 28.1–45.9) (p = 0.0014).
Table 4.
Prognostic factors for success (delivery)
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Male age at COS | 0.91 (0.83–0.999) | 0.047 | – | |
| Female age at COS | 0.87 (0.79–0.96) | 0.005 | 0.87 (0.79–0.96) | 0.005 |
| AMH | 1.31 (1.03–1.67) | 0.029 | – | |
| FSH | 0.92 (0.78–1.11) | 0.415 | ||
| Retrieved oocytes | 1.02 (0.96–1.09) | 0.466 | ||
| Spermatozoa | ||||
| Banked | 1 | |||
| Fresh | 2.49 (0.99–6.29) | 0.053 | – | |
| TESE | 1.48 (0.49–4.51) | 0.490 | – | |
Univariate logistic regression analysis. Factors with a p value less than 0.1 were submitted to multivariate logistic regression analysis. COS controlled ovarian stimulation
Discussion
Scientific literature about fertility in male survivors of haematological cancer included studies on sperm damage caused by various cancer treatment protocols [23], on the rate of utilisation of banked samples [10], and on the results of ART using cryopreserved sperm [24] or testicular-retrieved sperm [25]. This is a heterogeneous population suffering from different types of cancer, which occur at various ages (the age of onset in our population ranges between 2.6 and 49.5 years). Whenever possible, sperm cryopreservation is certainly an effective solution. Approximately 15% of our male cancer survivors who desired fertility were unable to have biological children because of azoospermia and failed TESE. This highlights the need for adequate counselling about fertility preservation prior to the initiation of chemotherapy, as these men could potentially have improved their chance of having biological children if they had banked sperm prior to chemotherapy. However, only a small percentage of these patients use cryopreserved sperm [10]. Recent national cohort studies on male cancer survivors born during 1960 and 1998 [26] and 1965–1985 [27] showed a significant reduction in marriage and paternity and an increased use of assisted reproduction for men with haematological cancers, stressing the life-long impact of their earlier illness on social behaviour and choices [26].
Our study aimed at highlighting some topics observed in new generations and rarely mentioned in the scientific papers published so far. The first issue refers to the extremely low paternity rate before cancer diagnosis. Of 97 patients who were older than 30 years old at diagnosis and were seen at our fertility centre to cryopreserve sperm or to achieve fatherhood, only 12.3% already had a child. This finding seems difficult to understand if we consider that in Italy, between 1982 and 1996, the paternity rate before stem cell transplantation for haematological cancer was 28% [21], while in Norway for cancer patients born between 1967 and 1978, it was 26% [22] and in European Hodgkin’s lymphoma survivors treated between 1974 to 2004, it was 48% (403 out 902) [9]. The only plausible explanation is that, over the last 20 years, the male age at first fatherhood has considerably increased in this selected population and that, at the time of being diagnosed with cancer, many men had not yet even considered becoming a parent. The Italian National Statistics Institute (ISTAT) report an average age of 30.8 years in women giving birth to their first child in 2015—the highest in Europe (where the average is 28.9 years)—and a mean age for men fathering a child of 35.3 years [28]. Interestingly, this reproductive scenario is similar to that of Spain and Greece, both southern European countries that have been particularly affected by the recent financial crisis, but also that of Switzerland and Luxembourg, whose socioeconomic situations are the complete opposite of that in southern Europe [29].
The second issue is the low spontaneous pregnancy rate occurring after surviving cancer (11.8% over 7–185 months of sexual intercourse, median 45.2 months), if compared to 61.6% reported by van der Kaaij et al. [9] on 334 Hodgkin’s lymphoma survivors with procreative intentions, treated between 1974 and 2004. We underline that only 38.7% of our patients wishing a child recovered sperm production and this can be explained by the high number of patients undergoing bone narrow transplantation (36 out of 93).
The third issue is the demonstration, in the last 20 years, of a progressive increase of female ageing in couples of cancer survivors with procreative desires. In our fertility centre, the median age of non-cancer-related women (n = 29,252) has been substantially stable over the last 20 years (36 years until 2007 and 37 years until 2008). On the other hand, the partners of male cancer survivors were clearly younger (average age 32.2 years up to 2007 and 34.2 years up to 2011), in line with data published by other investigators [24, 30–32]. Starting in 2012, the average women’s age has increased reaching that of the general infertile population. This change in reproductive demographics has been associated with an increase in ART requests after antineoplastic treatments in male haematological cancer survivors, when compared to the general population [27]. In our study, ART procedures were used by almost 60% of patients with reproductive intentions.
We want to emphasise that our data refer to ICSI cycles carried out with cryopreserved ejaculated or thawed testicular sperm and also with fresh sperm in patients with oligo-astheno-teratozoospermia who did not bank sperm prior to cancer treatment or, for cases of purely female fertility determined by age and reduced ovarian function [33].
The cumulative success rate of ICSI was high. Van der Kaaij et al. [9] reported 62% success rate per couple in Hodgkin’s lymphoma survivors, while Babb et al. (2012) [8] reported 18 successful pregnancies in 25 patients (72%) undergoing stem cell transplant (SCT) for acute or chronic myeloid leukaemia. However, female age was not reported. We found only four studies [24, 30–32] reporting average female age at the time of assisted reproduction, being respectively 34.3, 34.8, 31.2, and 32 years, far below our data (women’s average age 37. 0 ± 4. 7 years). If we consider that in our population 1 in 5 female partners were aged ≥ 40 at the time of COS, and almost 1 in 3 had a poor ovarian reserve, according to the Bologna criteria [19], it is easy to understand why our cumulative delivery rate per couple is lower (43.6%). Because delayed childbearing/female ageing is progressively increasing since the 2000s, the ART success rates described in past decades are no longer applicable and this should be discussed with cancer patients before sperm cryopreservation.
The major limitation of this study is the low number of patients performing ART, although it represents one of the biggest case histories for haematological cancer survivors [6, 8, 9, 24, 25, 30–32, 34]. Another limitation of this study is the presence of different forms of lymphatic or haematological malignancies and no detailed discussion of the chemotherapy regimens used and their disparate effects on fertility. Another limitation of our study is that ART results were limited by the introduction of Italy Law 40 of 2004, banning the cryopreservation of embryos during the 2004–2009 period and that, even today, narrowing the use of sperm and oocytes from donors, driving couples to resort to IVF in other countries [20]. Finally, our study might reflect a socio-economic condition limited to southern Europe.
In summary, this study offers new insight into reproductive habits of haematological cancer survivors and the risks of childless linked to delayed childbearing. As a result, we think this topic should be taken into consideration by national health institutions and scientific and patient associations.
Acknowledgments
The authors thank Pasquale Patrizio, M.D., M.B.E., Yale University, Fertility Center, for helping in preparing this manuscript.
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Botchan A, Karpol S, Lehavi O, Paz G, Kleiman SE, Yogev L, Yavetz H, Hauser R. Preservation of sperm of cancer patients: extent of use and pregnancy outcome in a tertiary infertility center. Asian J Androl. 2013;15(3):382–386. doi: 10.1038/aja.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotaling JM, Lopushnyan NA, Davenport M, Christensen H, Pagel ER, Muller CH, Walsh TJ. Raw and test-thaw semen parameters after cryopreservation among men with newly diagnosed cancer. Fertil Steril. 2013;99(2):464–469. doi: 10.1016/j.fertnstert.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 3.Ethics ETFo, Law Taskforce 7: ethical considerations for the cryopreservation of gametes and reproductive tissues for self use. Hum Reprod. 2004;19(2):460–462. doi: 10.1093/humrep/deh051. [DOI] [PubMed] [Google Scholar]
- 4.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN, Brennan LV, Oktay K, American Society of Clinical Oncology American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24(18):2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 5.Daudin M, Rives N, Walschaerts M, Drouineaud V, Szerman E, Koscinski I, Eustache F, Saïas-Magnan J, Papaxanthos-Roche A, Cabry-Goubet R, Brugnon F, le Lannou D, Barthélémy C, Rigot JM, Fréour T, Berthaut I, Giscard d'Estaing S, Touati F, Mélin-Blocquaux MC, Blagosklonov O, Thomas C, Benhamed M, Schmitt F, Kunstmann JM, Thonneau P, Bujan L. Sperm cryopreservation in adolescents and young adults with cancer: results of the French national sperm banking network (CECOS) Fertil Steril. 2015;103(2):478–486. doi: 10.1016/j.fertnstert.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Chan PT, Palermo GD, Veeck LL, Rosenwaks Z, Schlegel PN. Testicular sperm extraction combined with intracytoplasmic sperm injection in the treatment of men with persistent azoospermia postchemotherapy. Cancer. 2001;92(6):1632–1637. doi: 10.1002/1097-0142(20010915)92:6<1632::AID-CNCR1489>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 7.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340(8810):17–18. doi: 10.1016/0140-6736(92)92425-F. [DOI] [PubMed] [Google Scholar]
- 8.Babb A, Farah N, Lyons C, Lindsay K, Reddy N, Goldman J, Apperley JF, Salooja N. Uptake and outcome of assisted reproductive techniques in long-term survivors of SCT. Bone Marrow Transplant. 2012;47(4):568–573. doi: 10.1038/bmt.2011.134. [DOI] [PubMed] [Google Scholar]
- 9.van der Kaaij MA, van Echten-Arends J, Heutte N, Meijnders P, Abeilard-Lemoisson E, Spina M, Moser EC, Allgeier A, Meulemans B, Lugtenburg PJ, Aleman BM, Noordijk EM, Fermé C, Thomas J, Stamatoullas A, Fruchart C, Eghbali H, Brice P, Smit WG, Sebban C, Doorduijn JK, Roesink JM, Gaillard I, Coiffier B, Lybeert ML, Casasnovas O, André M, Raemaekers JM, Henry-Amar M, Kluin-Nelemans JC, European Organisation for Research and Treatment of Cancer Lymphoma Group and the Groupe d’Étude des Lymphomes de l'Adulte Cryopreservation, semen use and the likelihood of fatherhood in male Hodgkin lymphoma survivors: an EORTC-GELA Lymphoma Group cohort study. Hum Reprod. 2014;29(3):525–533. doi: 10.1093/humrep/det430. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari S, Paffoni A, Filippi F, Busnelli A, Vegetti W, Somigliana E. Sperm cryopreservation and reproductive outcome in male cancer patients: a systematic review. Reprod BioMed Online. 2016;33(1):29–38. doi: 10.1016/j.rbmo.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 3. Cambridge: Published on behalf of the World Health Organization by Cambridge University Press; 1992. [Google Scholar]
- 12.World Health Organization . WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4. Cambridge: Published on behalf of the World Health Organization by Cambridge University Press; 1999. [Google Scholar]
- 13.World Health Organization DoRHaR . WHO laboratory manual for the examination and processing of human semen. Fifth edition ed 2010. [Google Scholar]
- 14.Ziegler WF, Chapitis J. Human motile sperm recovery after cryopreservation: freezing in nitrogen vapor vs the direct plunge technique. Prim Care Update Ob Gyns. 1998;5(4):170. doi: 10.1016/S1068-607X(98)00072-9. [DOI] [PubMed] [Google Scholar]
- 15.Negri L, Albani E, DiRocco M, Morreale G, Novara P, Levi-Setti PE. Testicular sperm extraction in azoospermic men submitted to bilateral orchidopexy. Hum Reprod. 2003;18(12):2534–2539. doi: 10.1093/humrep/deg497. [DOI] [PubMed] [Google Scholar]
- 16.Hauser R, Botchan A, Amit A, Ben Yosef D, Gamzu R, Paz G, Lessing JB, Yogev L, Yavetz H. Multiple testicular sampling in non-obstructive azoospermia--is it necessary? Hum Reprod. 1998;13(11):3081–3085. doi: 10.1093/humrep/13.11.3081. [DOI] [PubMed] [Google Scholar]
- 17.Hendriks DJ, Mol BW, Bancsi LF, Te Velde ER, Broekmans FJ. Antral follicle count in the prediction of poor ovarian response and pregnancy after in vitro fertilization: a meta-analysis and comparison with basal follicle-stimulating hormone level. Fertil Steril. 2005;83(2):291–301. doi: 10.1016/j.fertnstert.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 18.La Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio AC, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum Reprod Update. 2010;16(2):113–130. doi: 10.1093/humupd/dmp036. [DOI] [PubMed] [Google Scholar]
- 19.Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L, et al. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616–1624. doi: 10.1093/humrep/der092. [DOI] [PubMed] [Google Scholar]
- 20.Levi Setti PE, Albani E, Matteo M, Morenghi E, Zannoni E, Baggiani AM, Arfuso V, Patrizio P. Five years (2004-2009) of a restrictive law-regulating ART in Italy significantly reduced delivery rate: analysis of 10,706 cycles. Hum Reprod. 2013;28(2):343–349. doi: 10.1093/humrep/des404. [DOI] [PubMed] [Google Scholar]
- 21.Anserini P, Chiodi S, Spinelli S, Costa M, Conte N, Copello F, Bacigalupo A. Semen analysis following allogeneic bone marrow transplantation. Additional data for evidence-based counselling. Bone Marrow Transplant. 2002;30(7):447–451. doi: 10.1038/sj.bmt.1703651. [DOI] [PubMed] [Google Scholar]
- 22.Magelssen H, Melve KK, Skjaerven R, Fossa SD. Parenthood probability and pregnancy outcome in patients with a cancer diagnosis during adolescence and young adulthood. Hum Reprod. 2008;23(1):178–186. doi: 10.1093/humrep/dem362. [DOI] [PubMed] [Google Scholar]
- 23.Leader A, Lishner M, Michaeli J, Revel A. Fertility considerations and preservation in haemato-oncology patients undergoing treatment. Br J Haematol. 2011;153(3):291–308. doi: 10.1111/j.1365-2141.2011.08629.x. [DOI] [PubMed] [Google Scholar]
- 24.Muller I, Oude Ophuis RJ, Broekmans FJ, Lock TM. Semen cryopreservation and usage rate for assisted reproductive technology in 898 men with cancer. Reprod BioMed Online. 2016;32(2):147–153. doi: 10.1016/j.rbmo.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Dar S, Orvieto R, Levron J, Haas J, Gat I, Raviv G. IVF outcome in azoospermic cancer survivors. Eur J Obstet Gynecol Reprod Biol. 2018;220:84–87. doi: 10.1016/j.ejogrb.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Pivetta E, Maule MM, Pisani P, Zugna D, Haupt R, Jankovic M, Arico M, Casale F, Clerico A, Cordero di Montezemolo L, Kiren V, Locatelli F, Palumbo G, Pession A, Pillon M, Santoro N, Terenziani M, Valsecchi MG, Dama E, Magnani C, Merletti F, Pastore G, for the Italian Association of Pediatric Hematology and Oncology (AIEOP) Group Marriage and parenthood among childhood cancer survivors: a report from the Italian AIEOP Off-Therapy Registry. Haematologica. 2011;96(5):744–751. doi: 10.3324/haematol.2010.036129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunnes MW, Lie RT, Bjorge T, Ghaderi S, Ruud E, Syse A, et al. Reproduction and marriage among male survivors of cancer in childhood, adolescence and young adulthood: a national cohort study. Br J Cancer. 2016;114(3):348–356. doi: 10.1038/bjc.2015.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ISTAT. Births and fertility among the resident population 2015 [Available from: http://www.istat.it/en/files/2016/11/EN_Births_Fertility_2015.pdf?title=Birth+and+fertility+-+28+Nov+2016+-+Full+text.pdf.
- 29.Newsrelease E. Birth and fertility, over 5 million babies born in the EU in 2015. Women first became mothers at almost 29 on average 2017 [Available from: http://ec.europa.eu/eurostat/documents/2995521/7898237/3-08032017-AP-EN.pdf/b17c1516-faad-4e65-b291-187826a7ac88.
- 30.Garcia A, Herrero MB, Holzer H, Tulandi T, Chan P. Assisted reproductive outcomes of male cancer survivors. J Cancer Surviv. 2015;9(2):208–214. doi: 10.1007/s11764-014-0398-7. [DOI] [PubMed] [Google Scholar]
- 31.Hourvitz A, Goldschlag DE, Davis OK, Gosden LV, Palermo GD, Rosenwaks Z. Intracytoplasmic sperm injection (ICSI) using cryopreserved sperm from men with malignant neoplasm yields high pregnancy rates. Fertil Steril. 2008;90(3):557–563. doi: 10.1016/j.fertnstert.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 32.van Casteren NJ, van Santbrink EJ, van Inzen W, Romijn JC, Dohle GR. Use rate and assisted reproduction technologies outcome of cryopreserved semen from 629 cancer patients. Fertil Steril. 2008;90(6):2245–2250. doi: 10.1016/j.fertnstert.2007.10.055. [DOI] [PubMed] [Google Scholar]
- 33.Bhattacharya S, Maheshwari A, Mollison J. Factors associated with failed treatment: an analysis of 121,744 women embarking on their first IVF cycles. PLoS One. 2013;8(12):e82249. doi: 10.1371/journal.pone.0082249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ragni G, Somigliana E, Restelli L, Salvi R, Arnoldi M, Paffoni A. Sperm banking and rate of assisted reproduction treatment: insights from a 15-year cryopreservation program for male cancer patients. Cancer. 2003;97(7):1624–1629. doi: 10.1002/cncr.11229. [DOI] [PubMed] [Google Scholar]