Fig. 2.
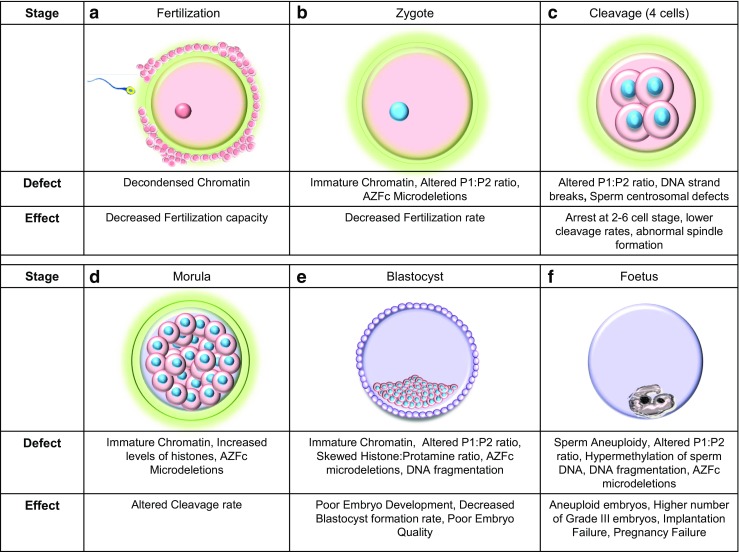
Genetic factors of paternal origin that impact embryo quality. Images a to e depict the stages in human embryonic development. a Structural defects of sperm chromatin can decrease the fertilization capacity of sperm. b The rate at which the sperm fertilizes the oocyte can be affected by sperm chromatin defects, alterations in the protamine ratios, and the [Azoospermia factor c] AZFc microdeletions in the Y chromosome. c At the 4 cell stage, the zygotic genome is activated and dependence on maternal genome is eliminated. Embryonic development may be arrested at this stage if the P1/P2 ratio is unbalanced. d The morula stage [16–32 cells] is characterized by a series of cleavage events that successively generate smaller cells. Structural chromatin defects and an increased level of histones can decelerate the cleavage rate with the presence of Y chromosome microdeletions can increase the cleavage rate. e The blastocyst stage that is characterized by the presence of the trophectoderm and the inner cell mass. A skewed histone to protamine ration [HPR] can decrease blastocyst formation rates, poor embryonic development is seen when the embryo is fertilized by sperm carrying chromatin defects and altered P1/P2 ratios. Poor quality embryos are noted when there is an altered protamine 1/protamine 2 ratio and the paternal Y chromosome harbors and AZFc microdeletions. In ART, embryo transfer is performed at this stage of embryonic development. f The growth of the fetus after implantation into the endometrium of the uterus. Presence of altered P1/P2 ratios can cause implantation failure while fertilization by aneuploid sperm, altered protamine ratios, and sperm epigenetic defects can lead to failure to establish pregnancy
