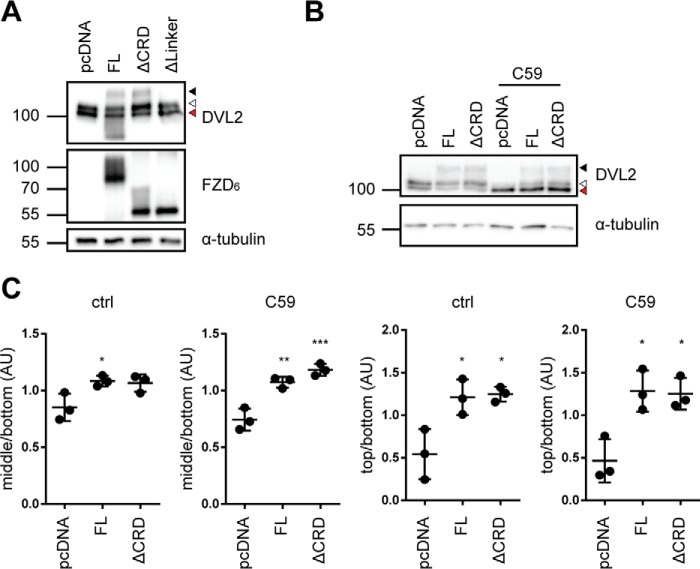
Figure 3.
DVL2 signaling induced by FZD6 deletion mutants. A, HEK293T cells were transfected with empty vector (pcDNA), full-length FZD6 (FL), ΔCRD-FZD6 (ΔCRD), and ΔLinker-FZD6 (ΔLinker). Cell lysates were analyzed for the FZD-induced electrophoretic mobility shift from unshifted (fastest-migrating, bottom band; red triangle), shifted (open triangle), and hypershifted (filled triangle) DVL2 using an anti-DVL2 antibody to detect endogenously expressed DVL2. Expression of FZD constructs was detected by an anti-FZD6 antibody; anti-α-tubulin antibody was used as a loading control. B, HEK293T cells were transfected with empty vector (pcDNA), full-length FZD6 (FL), and ΔCRD-FZD6 (ΔCRD) and kept overnight in the absence or presence of C59. Cell lysates were analyzed for the FZD-induced electrophoretic mobility shift from unshifted (fastest-migrating band; red triangle), shifted (open triangle), and hypershifted (filled triangle) DVL2 using an anti-DVL2 antibody to detect endogenously expressed DVL2. C, the three prominent DVL2 bands were quantified by densitometry. Bottom band, unshifted (red triangle); middle band, shifted (open triangle); top band, hypershifted (filled triangle). The scatter dot plot represents normalized values from three independent experiments for the mean ratio of middle/bottom or top/bottom bands with S.D. Tubulin detection was not included in the calculations. For details, see “Experimental procedures.” Statistical analysis was done by one-way ANOVA with Tukey's multiple comparisons post-test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.