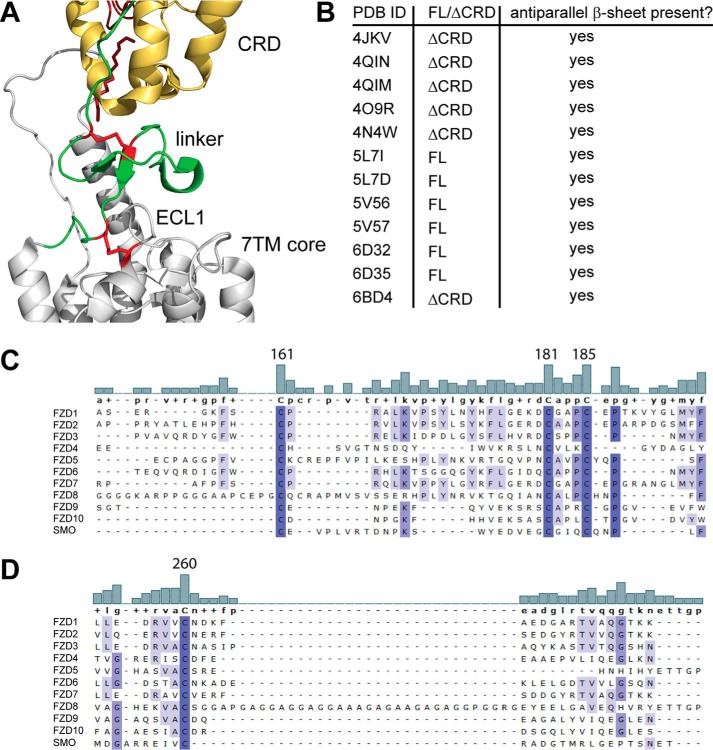
Figure 5.
Identification of a well-conserved triad of cysteines in the linker domain. A, close-up of the SMO structure shown in Fig. 1A. CRD, yellow; linker, green; 7TM core, gray. In addition, the linker domain cysteines corresponding to human FZD6 Cys-161, Cys-181, and Cys-185 and Cys-260 in ECL1 are shown as red sticks. B, the table summarizes information about the presence of the antiparallel β-sheet in the linker domain from all published SMO crystal structures and one FZD4 structure (PDB code 6BD4). FL, CRD present; ΔCRD, CRD absent. C and D, alignment of the extracellular linker domains and ECL1 of all human class F receptor homologs shows a high degree of conservation among the cysteines in the linker domain (C) and the cysteine in ECL1 (D). The bar graphs show the degree of conservation between the compared sequences. Numbers identify Cys-161, Cys-181, and Cys-185 in human FZD6. Increasing intensity of blue indicates a higher degree of conservation. Alignment was done using MAFFT with default settings. Structures were rendered using PyMOL (PyMOL Molecular Graphics System, version 2.0, Schrödinger, LLC).