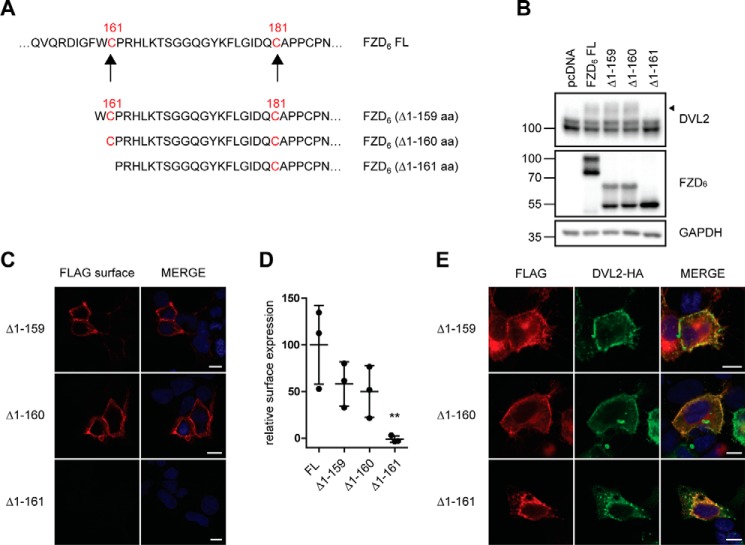
Figure 6.
Cys-161 defines the minimal length of a functional FZD6 construct. A, schematic presentation of FZD6 ΔCRD constructs with differences in the N-terminal amino acid. The cysteines in the linker domain are highlighted in red. B, HEK293T cells were transfected with empty vector (pcDNA), full-length FZD6 (FL), and N-terminal deletion mutants. The electrophoretic mobility shift of endogenous DVL2 was assessed by immunoblotting using an anti-DVL2 antibody. The filled triangle marks the band of hypershifted DVL2 induced by FZD overexpression. Protein expression was verified with an anti-FZD6 antibody; anti-GAPDH was used as a loading control. C, HEK293T cells were transfected with various N-terminal deletion mutants. Confocal photomicrographs of HEK293T cells transiently expressing truncated FZD6 with indirect immunofluorescence using fluorophore-labeled secondary antibody against the anti-FLAG antibody on nonpermeabilized cells (FLAG surface). D, surface expression of the transfected FZD6 constructs was quantified using a cell ELISA based on detection of anti-FLAG under nonpermeabilized conditions. The scatter dot plot represents means with S.D. of three independent experiments performed in triplicates. Statistical analysis was done by one-way ANOVA. Significance levels denoting differences between FL FZD6 and mutant receptor surface expression are given as follows: **, p < 0,01. E, confocal photomicrographs of DVL2 recruitment in HEK293T cells transfected with FZD6 N-terminal deletion mutants and DVL2-HA and stained with anti-FLAG (red, FZD6) and anti-HA (green, DVL2) antibodies. Scale bars = 10 μm.