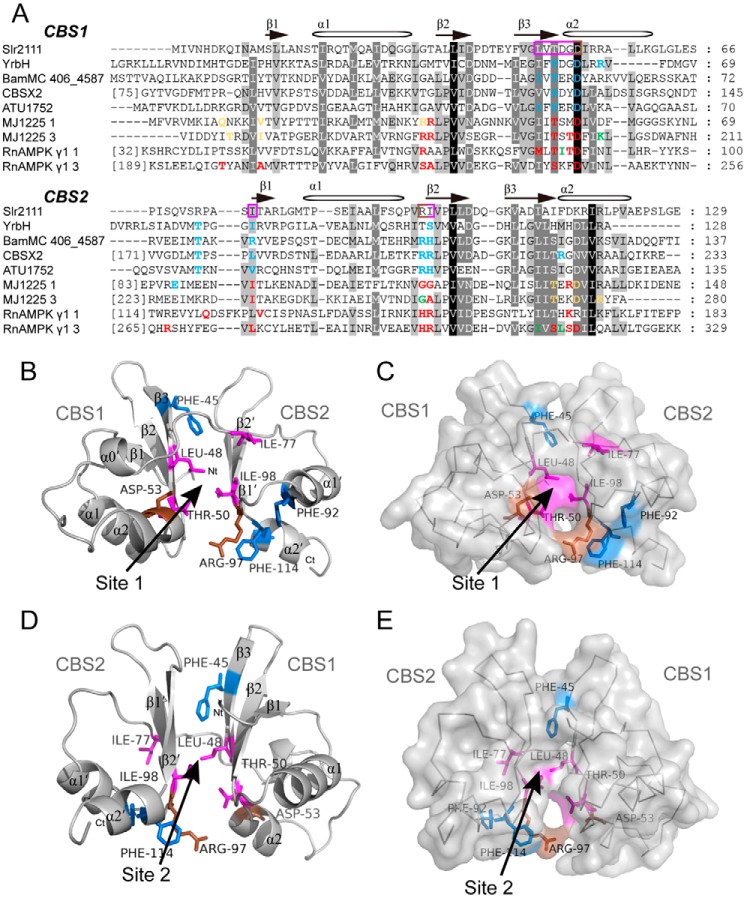
Figure 2.
Sequence alignment of CBS domains and model building of Slr2111. A, sequence alignments were performed by GeneDoc. Nucleotide-binding sites are colored: green, amino acids binding ATP; blue, amino acids binding AMP; yellow, amino acids binding ADP; amino acids binding more than two kinds of nucleotides are shown in red. Possible binding sites of Slr2111 are highlighted in a violet frame. B–E, simulation of the Slr2111 structure (10–123 aa). The three-dimensional model was obtained from the SWISS-MODEL server, and a putative signal-transduction protein with CBS domains from B. ambifaria MC40–6 (PDB code 4fry) was used as reference structure (see “Materials and methods” for details). Side chains of aromatic residues are indicated for three phenylalanine residues (Phe-45, Phe-92, and Phe-114) (blue). Leu-48, Thr-50, Ile-77, and Ile-98 are shown in violet, Asp-53 and Arg-97 are shown in brown. D and E depicts the structure after rotation around 180 degrees of B and C, respectively. Two possible binding sites of adenine nucleotides are indicated. Image was generated in PyMOL.