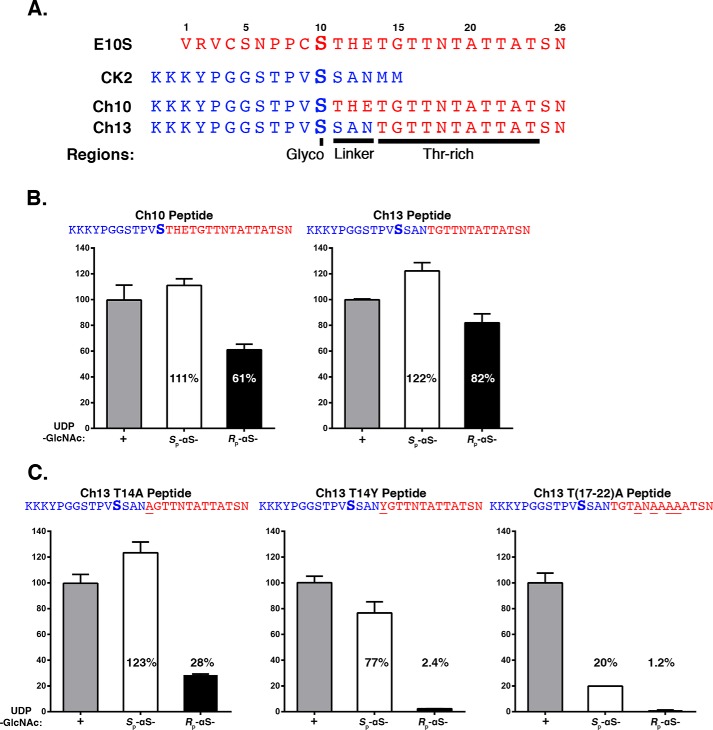
Figure 3.
The HCF-1PRO-repeat threonine-rich region is responsible for the relaxed E10S peptide UDP-GlcNAc co-substrate requirements. A, amino acid sequences of native E10S and CK2 peptides as well as two chimeric peptides derived from CK2-E10S fusion (Ch10 and Ch13) are shown. The serine for glycosylation (Glyco) is depicted in boldface type. The linker and Thr-rich region are indicated. B and C, in vitro peptide glycosylation assays were performed using Ch10 and Ch13 chimeric peptides (B) and Ch13 T14A, T14Y, and T(17–22)A mutant peptides (C). Peptides were incubated with WT OGT and either UDP-GlcNAc, Sp-αS-UDP-GlcNAc, or Rp-αS-UDP-GlcNAc, as indicated. Peptide glycosylation was detected using LC-MS. O-GlcNAcylation of the different peptides was in each case normalized to O-GlcNAcylation activity with UDP-GlcNAc as follows: Ch10, 3.2 × 108; Ch13 WT, 1.6 × 108; Ch13 T14A, 3.6 × 108; Ch13 T14Y, 2.0 × 108; and Ch13 T(17–22)A, 1.5 × 107. Error bars, S.D.