Figure 4.
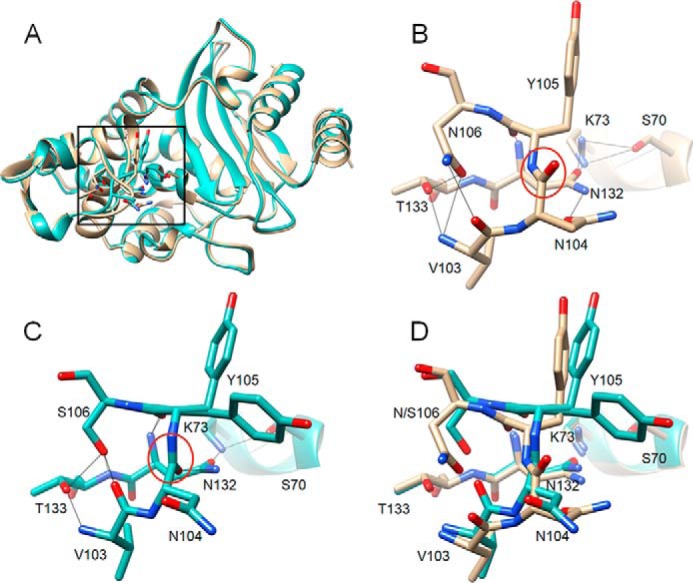
Alignment of structures of WT CTX-M-14 and N106S mutant. A, ribbon diagram of alignment of structures of WT CTX-M-14 (PDB code 1YLT) (9) (tan) and N106S-chain B (green). Side chains for residues 103–106, 132–133, and 70, 73 are shown as sticks. The box indicates the region of the structure shown in B–D. B, WT CTX-M-14 residues 103–106, 132–133, and 70, 73 are shown as sticks and labeled. Hydrogen bonds are indicated by black lines. The carbonyl oxygen of the peptide bond between positions Asn-104 and Tyr-105 is pointed upwards away from Asn-132 and circled in red. C, N106S structure with residues 103–106, 132–133, and 70, 73 shown as sticks and labeled. Hydrogen bonds are indicated by black lines. The carbonyl oxygen of the peptide bond between positions Asn-104 and Tyr-105 is circled in red and is pointed downwards forming a hydrogen bond with the side chain of residue Asn-132. The side chain of residue Asn-104 is rotated away from Asn-132 and out of the active site. The Tyr-105 side chain exists in two conformations in the structure. D, alignment of 103–106, 132–133, and 70, 73 from the WT CTX-M-14 (tan) and N106S (green) structures. In all panels, oxygen and nitrogen atoms are colored red and blue, respectively.
