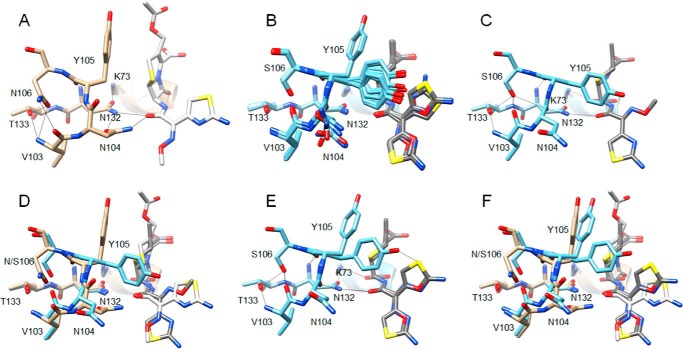
Figure 5.
Comparison of the CTX-M-14 S70G structure in complex with cefotaxime (PDB code 4PM5) (21) with that of the S70G/N106S enzyme in complex with CTX. A, S70G/CTX structure (tan) showing residues 103–106, 132–133, and 70, 73 as sticks and labeled. Hydrogen bonds are indicated by black lines. Note that the carbonyl oxygen of the peptide bond between positions Asn-104 and Tyr-105 is pointed upwards away from Asn-132, whereas the Asn-104 side chain makes hydrogen bonds to Asn-132 and the acyl-amide group of cefotaxime. B, alignment of residues 103–106, 132–133, and 70, 73 and cefotaxime from the eight molecules in the asymmetric unit of the S70G/N106S/CTX (light blue) structure. Cefotaxime is colored dark gray. The peptide bond between Asn-104 and Tyr-105 has a flipped orientation compared with A for all eight molecules. C, S70G/N106S/CTX-chain A structure showing residues 103–106, 132–133, and 70, 73 as sticks and labeled. Note that the carbonyl oxygen of the peptide bond between positions Asn-104 and Tyr-105 is pointed down and forms a hydrogen bond with Asn-132, whereas the Asn-104 side chain is rotated out of the active site, and no longer forms a hydrogen bond with the acyl-amide group of cefotaxime. D, alignment of residues 103–106, 132–133, and 70, 73 and cefotaxime from S70G/CTX (tan) and S70G/N106S/CTX-chain A (light blue) structures. Cefotaxime from the S70G structure is colored white and that from S70G/N106S is dark gray. E, S70G/N106S/CTX-chain C (light blue) structure showing residues 103–106, 132–133, and 70, 73 as sticks and labeled. Note the altered conformations of the Tyr-105 and Asn-104 side chains compared with chain A shown in C and the dual conformations of the oxime moiety and aminothiazole ring of cefotaxime. F, alignment of residues 103–106, 132–133, and 70, 73 and cefotaxime from S70G/CTX (tan) and S70G/N106S/CTX-chain C (light blue) structures. Cefotaxime from the S70G structure is colored white and that from S70G/N106S is dark gray.