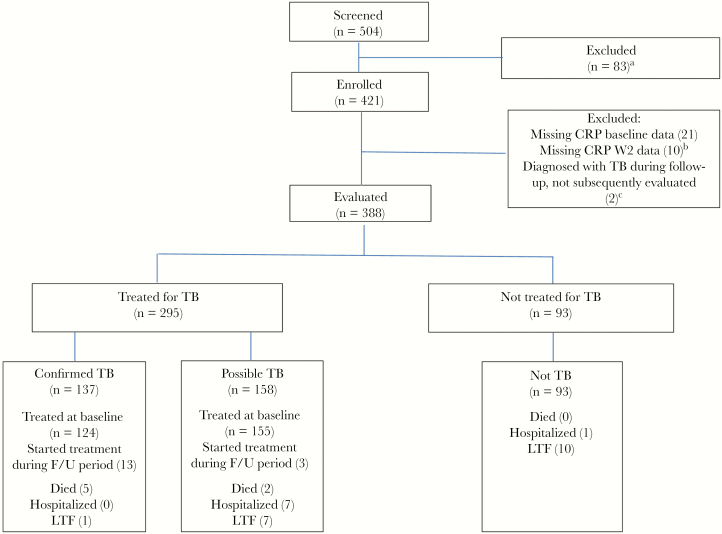
Figure 1.
Participant outcomes during the 8-week follow-up period. aNot able to attend for regular review (28), no active symptoms (17), alternative medical diagnosis (14), KPS <40 (5), pneumocystis pneumonia (4), informed consent not obtained (3), sputum smear positive (3), already on antituberculosis treatment (3), other (6). bConfirmed TB (4), possible TB (2), not TB (3), TB diagnosed on culture not treated at BL visit (1). cDied before antituberculosis treatment initiation (1), referred for inpatient Rx before re-baselined week 2 visit (1). Abbreviations: CRP, C-reactive protein; F/U, follow-up; LTF, lost to follow-up; TB, tuberculosis.