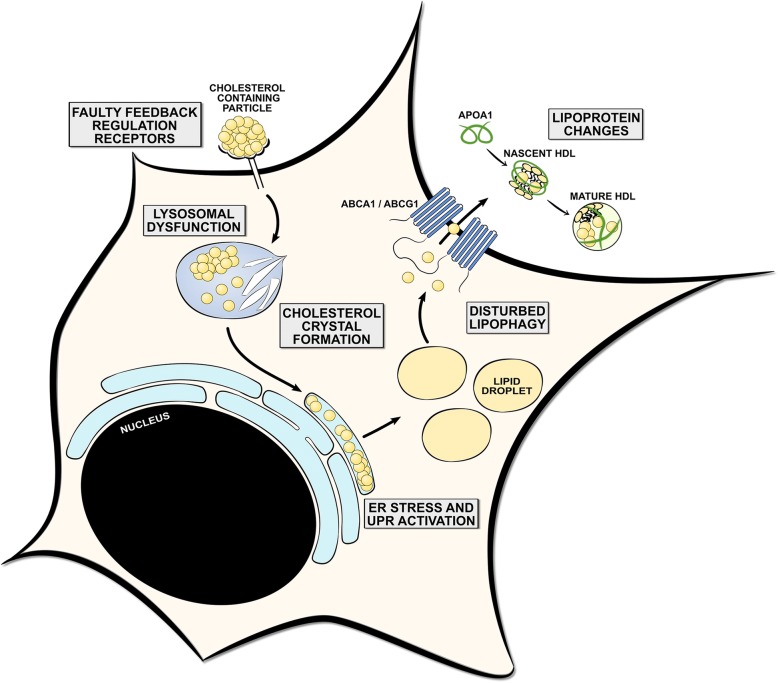
Fig. 4.
Homeostatic and dysfunctional processing of cholesterol-containing lipid particles. During homeostasis, phagocytes are well equipped to cope with relatively minor increases of cholesterol. However, sustained intracellular accumulation of cholesterol, as observed in in many peripheral pathologies and following infections with persistent pathogens, can lead to disturbances in pathways that mediate the degradation, storage, or efflux of cholesterol. First, faulty feedback regulation of phagocytic receptors may result in an uncontrolled uptake of cholesterol-containing lipid particles. Second, lysosomal cholesterol accumulation can result in lysosomal dysfunction by reducing lysosomal acidification and causing lysosomal leakiness. In addition, sustained accumulation of cholesterol can lead to the formation of cholesterol crystals that activate the caspase-1-activating NLRP3 inflammasome. Third, persistent cholesterol trafficking to ER membranes can trigger ER stress and the unfolded protein response (UPR). Fourth, dysfunctional lipophagy machinery can hamper the capacity of foamy phagocytes to process cholesterol within lipid droplets, thereby impeding the cells’ capacity to dispose of intracellular cholesterol. Finally, quantitative and qualitative changes in lipoproteins can impact the capacity of foamy phagocytes to efflux cholesterol. Altogether, disturbances in the abovementioned pathways are well-known to promote the induction of a disease-promoting phenotype of foamy phagocytes and eventually even cause apoptosis. While ample evidence suggests that faulty regulation of these pathways also occurs in myelin-containing phagocytes, more research is warranted to define to what extent they impact their inflammatory features