Key Teaching Points.
-
•
Catheter ablation treatment of post–heart transplantation atrial tachycardia (AT) can be challenging. The approach demands precise electroanatomic mapping and careful evaluation of the arrhythmia mechanism in the context of complex scar substrates that predispose to multiple ATs.
-
•
Multiple ATs in different cardiac atria with 3 different mechanisms could be observed in the same patient after orthotopic cardiac transplantation.
-
•
High-resolution/-density electroanatomic mapping using a multipolar catheter with small and closely spaced electrodes may better characterize the mechanisms of these challenging tachycardias and lead to successful ablation outcome.
Introduction
Atrial tachycardia (AT) after orthotopic heart transplantation (OHT) can be complex, occurring in the recipient as well as in the donor atria. Catheter ablation of these multiple ATs is challenging because of the complexity of atrial scar substrates. This case report demonstrates the merit of using a novel multipolar high-density mini-basket mapping catheter for fast, high-resolution mapping of 3 different ATs occurring in both atria of the orthotopic transplant heart.
Case report
A 55-year-old man with nonischemic dilated cardiomyopathy and refractory heart failure underwent OHT with biatrial anastomosis techniques in 1986 at age 25 years. He developed a symptomatic persistent AT and was referred for catheter ablation. He previously had undergone cavotricuspid isthmus (CTI) linear ablation for refractory right atrial (RA) flutter 3 years before onset of the latest AT. Twelve-lead surface electrocardiography of the AT showed positive P-wave morphology in lead V1 with a P-P interval of 490 ms. Transthoracic echocardiography showed moderately impaired left ventricular systolic function (ejection fraction 37%). There was no sign of heart transplant rejection. He was taking bisoprolol as the only antiarrhythmic medication.
Electrophysiological study and ablation
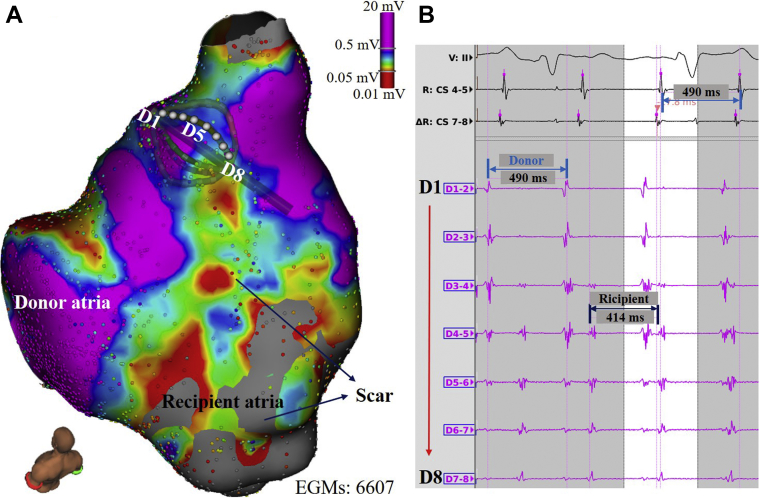
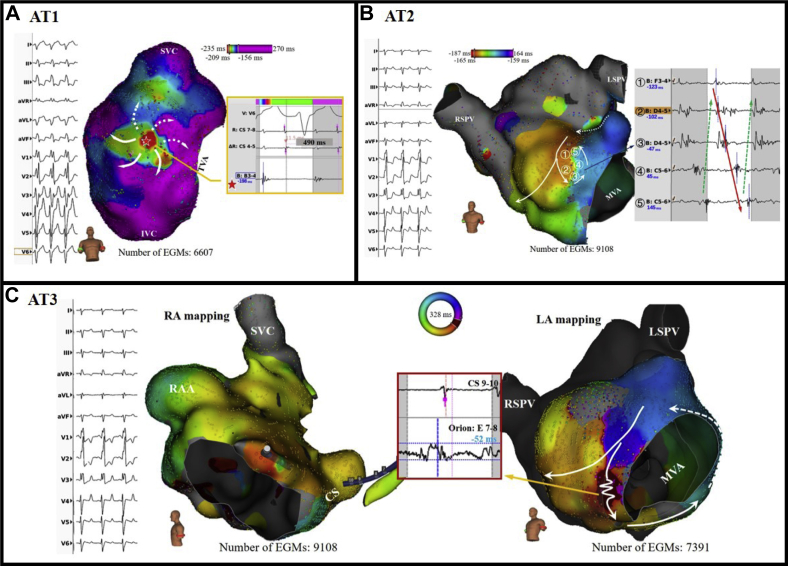
After obtaining written informed consent, an electrophysiological study was performed with the patient under general anesthesia. A 6F steerable 10-pole catheter positioned within the coronary sinus (CS) via the left femoral vein showed a proximal to distal CS activation pattern with a tachycardia cycle length (TCL) of 490 ms (AT1). A multipolar mini-basket mapping catheter (IntellaMap Orion, Boston Scientific, Cambridge, MA) was positioned in the RA for electroanatomic mapping. The multipolar catheter mapping showed donor and recipient atrial beats independently, with clearly demarcated activation patterns along the anastomotic suture line (Figure 1). The activation map of AT1 showed a focal AT at the donor RA anterolateral wall near the ostium of the RA appendage (Figure 2A). Radiofrequency ablation delivered from a 3.5-mm irrigated-tip catheter (IntellaNav, Boston Scientific, Marlborough, MA) at the earliest activation site resulted in acceleration of the tachycardia before termination of AT1. Given the “warm-up” phenomena, one might speculate that automaticity was the attributing mechanism of AT1.
Figure 1.
Independent recipient and donor atrial activations. A: Voltage map of the recipient and donor right atria guided by the multipolar mini-basket catheter. Scar areas are denoted by the color spectrum bar (bipolar voltage <0.5 mV). B: Multipolar intracardiac electrograms (EGMs) showing the recipient and donor atrial tachycardia independently. D1-2 shows the atrial tachycardia (AT) of the donor atrium (tachycardia cycle length [TCL] 490 ms). D7-8 shows the AT of the recipient atrium (TCL 414 ms).
Figure 2.
Activation maps and mechanisms of 3 different atrial tachycardias (ATs). A: Activation map of focal AT1 (tachycardia cycle length [TCL] 490 ms) with earliest focal activation from the anterolateral wall of the donor right atrium (RA), indicated by the red star. Ablation at this site terminated AT1 immediately. B: Activation map of the microreentrant AT2 (TCL 297 ms) from the donor left atrium (LA). Bipolar fractionated electrograms obtained with the high-resolution/-density mapping using the mini-basket catheter shows the microreentrant circuit (①–⑤). The Rhythmia auto-annotated activation times are marked by the arrowed red line spanning the entire TCL. C: Biatrial activation map of the mitral isthmus dependent macroreentrant AT3 (TCL 328 ms), with the RA being the bystander with a breakout at the atrial septum. Linear ablation at the isthmus (a zone of slow conduction with high-frequency fractionated electrograms) between the anterior mitral annulus and a region of scar in the LA anterior wall terminated AT3. CS = coronary sinus catheter; EGM = electrogram; IVC = inferior vena cava; LSPV = left superior pulmonary vein; MVA = mitral valve annulus; RAA = right atrial appendage; RSPV = right superior pulmonary vein; SVC = superior vena cava; TVA = tricuspid valve annulus.
Programmed electrical stimulation from the CS initiated a second AT with TCL of 297 ms (AT2). The CS activation pattern was from distal to proximal, suggesting a left AT. The min-basket catheter was advanced into the left atrium (LA) via a steerable sheath (Agilis, St. Jude Medical, Saint Paul, MN) after a single transseptal puncture. Electroanatomic mapping showed a microreentrant circuit on the donor LA anterior wall (Figure 2B). Ablation at this site successfully terminated the tachycardia.
A third AT with TCL of 328 ms and proximal to distal CS activation pattern (AT3) was then induced. Biatrial electroanatomic mapping showed a macroreentrant perimitral AT, with the RA being the bystander (Figure 2C). Linear ablation at the isthmus between the anterior mitral annulus and a region of scar in the LA anterior wall terminated AT3. Differential pacing confirmed bidirectional block of the anterior mitral isthmus ablation line. Thereafter, no more tachycardia was inducible. The total procedure fluoroscopy time was 26 minutes, and the total ablation time was 24 minutes (16 ablations). The patient recovered from the procedure without any complications. The patient maintained sinus rhythm without any antiarrhythmia medications during a 1-year follow-up period, and follow-up echocardiography showed left ventricular systolic function had improved, with an ejection fraction of 45%.
Discussion
ATs are frequently observed in about 10%–20% of patients after OHT. They can coexist in the recipient and donor atria.1 Catheter ablation is an effective treatment of these ATs. The reported median time from OHT to ablation for AT is about 8 years.2 An understanding of the surgical anatomy with a clear illustration of the scar substrate and electrical activation patterns by 3-dimensional electroanatomic mapping allowed us to identify the mechanisms of these complex ATs and helped to achieve a successful ablation outcome.
Small and closely spaced electrodes are reported to better detect viable myocardial tissue in zones of low-voltage tissue compared to conventional catheters with larger electrodes. Such electrodes arrangement can improve electroanatomic mapping resolution and ablation outcomes.3, 4 To our knowledge, this is the first reported case of using the mini-basket multipolar catheter with such an electrode arrangement paired with the Rhythmia mapping system (Boston Scientific Corp, Cambridge, MA) to guide multiple AT ablation after OHT.
Pathology and mechanisms of AT after OHT
Previous studies showed that the pathogenesis of AT after OHT included relative autonomic denervation; preexisting atrial disease of the recipient’s heart before transplantation; and iatrogenic surgical scar substrates in both atria after transplantation.2 Although AT can be prevalent in the RA and/or LA in either bicaval or biatrial anastomoses. and the mechanisms of ATs have significant variations depending on the technique of donor-to-recipient anastomosis. Biatrial anastomosis creates a greater degree of scarred and low-voltage areas of slow conduction within the atria as a result of intra-atrial sutures lines that provide substrates for local reentry, whereas bicaval anastomosis avoids extensive intra-atrial suture lines and therefore results in a lower prevalence of local reentrant AT. Exclusion of the pulmonary veins and the posterior LA is thought to be responsible for the very low incidence of atrial fibrillation with either surgical method.2 Table 1 lists the single and serial cases of post-OHT AT ablations guided by 3-dimensional mapping system reported in the literature in the last decade.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 The majority of ATs in stable OHT patients can be attributed to macroreentrant tachycardia, including CTI-dependent flutter and scar-reentrant AT.1, 2 Focal AT can be observed originating in low-voltage or border zones adjacent to biatrial anastomosis suture lines.13 In our case, 3 different ATs with 3 different mechanisms were detected in both right and left donor atria, in addition to the CTI-dependent flutter successfully ablated previously.
Table 1.
Cases of atrial tachycardia ablation guided by 3-dimensional mapping after orthotropic heart transplant
| Author | Year | No. of patients | Mapping | Mechanisms | Acute success | Recurrence | Follow-up |
|---|---|---|---|---|---|---|---|
| Mouhoub et al5 | 2017 | 30 | EnSite∗ and CARTO† | CTI-dependent AFL (93%); perimitral AT (3%); focal AT (3%) on anterior RA suture line | 93% | 17% recurred, 13% death | 39 ± 26.8 months |
| Schratter et al6 | 2016 | 1 | NavX∗ | 1 CTI-dependent AFL; 2 ATs in the recipient’s atrium untreated | 100% | — | — |
| McKillop and Miles7 | 2016 | 1 | NavX | 1 focal AT cross the gap on RA suture line | 100% | 0% | — |
| Jin et al8 | 2015 | 1 | CARTO | 1 focal AT from RA | 100% | 0% | — |
| Yamada et al9 | 2014 | 1 | CARTO | 1 focal AT from donor LA | 100% | — | — |
| Nof et al2 | 2013 | 7 | CARTO | 4 RDC AT (3 RA, 1 LA) | 100% | 0% | 9 ± 5.5 years |
| 3 focal AT (2 RACT, 1 CS) | 100% | 20% | 4.8 ± 3.6 years | ||||
| Laksman et al10 | 2013 | 1 | CARTO | 2 independent ATs (1 donor RA and 1 recipient RA) | 100% | — | — |
| Penafiel-Verdu et al11 | 2012 | 1 | CARTO | 3 focal AT from recipient RA | 100% | — | — |
| Minamiguchi et al12 | 2012 | 1 | EnSite array | 1 focal AT from donor RA | 100% | 0% | 12 months |
| Elsik et al13 | 2012 | 16 | EnSite and CARTO | 4 microreentrant AT from interatrial suture scar 2 focal AT from donor RA |
100% | 0% | 34 ± 15 months |
| Makanjee et al14 | 2010 | 2 | CARTO | 2 perimitral macroreentrant AT | 100% | 0% | 3 months |
| Solheim et al15 | 2009 | 1 | CARTO | 1 focal AT from donor RA | 100% | 0% | 2 months |
| Vaseghi et al1 | 2008 | 24 | NavX and CARTO | 6 RA macroreentrant AT; 1 macroreentrant AT from donor LA; 2 perimitral macroreentrant AT | 100% | 2.1% | 48 months |
AFL = atrial flutter; AT = atrial tachycardia; CS = coronary sinus; CTI = cavotricuspid isthmus; LA = left atrium; RA = right atrium; RACT = right atrial crista terminalis; RDC = recipient-to-donor atria conduction.
Abbott, Saint Paul, MN.
Biosense Webster, Diamond Bar, CA.
Clinical implications
Appropriate mapping strategy in the evaluation of ATs in a transplant heart is a key factor in achieving successful outcome. Conventional mapping strategy may be challenging because of the high prevalence of regional atrial scar with fractionated multicomponent electrograms after OHT, which can limit accurate timing annotation. It is often difficult to perform and interpret entrainment and postpacing interval mapping due to a number of limitations, including lack of capture in areas of scar, need for high-output capture, and changes in the tachycardia in response to pacing.
Catheters with smaller electrodes and interelectrode spacing can better demonstrate myocardial tissue heterogeneity and record significantly higher bipolar voltage amplitude in low-voltage areas compared to catheters with larger size/interelectrode spacing.4 Previous experiences showed that the multipolar high-resolution catheter paired with the Rhythmia mapping system may be advantageous for mapping complex scar-related arrhythmia with its automated annotation system. Such a system can identify the critical scar zones between recipient and donor heart accurately and efficiently, especially given the independent electrical activation of the donor and recipient atrial tissue.
Conclusion
Catheter ablation treatment of post–heart transplantation AT can be challenging. The approach demands precise electroanatomic mapping and careful evaluation of the arrhythmia mechanism in the context of complex scar substrates that predispose to multiple ATs. High-density mapping strategy using a multipolar catheter with a small and closely spaced electrode arrangement may facilitate successful outcomes.
References
- 1.Vaseghi M., Boyle N.G., Kedia R., Patel J.K., Cesario D.A., Wiener I., Kobashigawa J.A., Shivkumar K. Supraventricular tachycardia after orthotopic cardiac transplantation. J Am Coll Cardiol. 2008;51:2241–2249. doi: 10.1016/j.jacc.2008.02.065. [DOI] [PubMed] [Google Scholar]
- 2.Nof E., Stevenson W.G., Epstein L.M., Tedrow U.B., Koplan B.A. Catheter ablation of atrial arrhythmias after cardiac transplantation: findings at EP study utility of 3-D mapping and outcomes. J Cardiovasc Electrophysiol. 2013;24:498–502. doi: 10.1111/jce.12078. [DOI] [PubMed] [Google Scholar]
- 3.Nuhrich J.M., Kaiser L., Akbulak R.O., Schaffer B.N., Eickholt C., Schwarzl M., Kuklik P., Moser J., Jularic M., Willems S., Meyer C. Substrate characterization and catheter ablation in patients with scar-related ventricular tachycardia using ultra high-density 3-D mapping. J Cardiovasc Electrophysiol. 2017;28:1058–1067. doi: 10.1111/jce.13270. [DOI] [PubMed] [Google Scholar]
- 4.Anter E., Tschabrunn C.M., Josephson M.E. High-resolution mapping of scar-related atrial arrhythmias using smaller electrodes with closer interelectrode spacing. Circ Arrhythm Electrophysiol. 2015;8:537–545. doi: 10.1161/CIRCEP.114.002737. [DOI] [PubMed] [Google Scholar]
- 5.Mouhoub Y., Laredo M., Varnous S., Leprince P., Waintraub X., Gandjbakhch E., Hebert J.L., Frank R., Maupain C., Pavie A., Hidden-Lucet F., Duthoit G. Catheter ablation of organized atrial arrhythmias in orthotopic heart transplantation. J Heart Lung Transplant. 2018;37:232–239. doi: 10.1016/j.healun.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Schratter A., Schirripa V., Kosiuk J., Koutalas E., Hindricks G., Bollmann A. Electroanatomical high-density mapping of different tachycardias in the right atrium after heart transplantation. HeartRhythm Case Rep. 2016;2:517–520. doi: 10.1016/j.hrcr.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKillop M.S., Miles W.M. An unusual atrial tachycardia in a cardiac transplant patient. J Cardiovasc Electrophysiol. 2016;27:878–880. doi: 10.1111/jce.12922. [DOI] [PubMed] [Google Scholar]
- 8.Jin Q., Pehrson S., Jacobsen P.K., Chen X. Mapping strategy for multiple atrial tachyarrhythmias in a transplant heart. BMC Cardiovasc Disord. 2015;15:38. doi: 10.1186/s12872-015-0031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada T., Kumar V., Kay G.N. Regularly irregular atrial tachycardia following an orthotopic heart transplant: what is the mechanism? J Cardiovasc Electrophysiol. 2014;25:105–106. doi: 10.1111/jce.12258. [DOI] [PubMed] [Google Scholar]
- 10.Laksman Z.W., Skanes A.C., Klein G.J., Manlucu J. Dual atrial tachycardia in a transplant heart: when is "in" really "out"? J Cardiovasc Electrophysiol. 2013;24:1428–1431. doi: 10.1111/jce.12244. [DOI] [PubMed] [Google Scholar]
- 11.Penafiel-Verdu P., Salar-Alcaraz M., Fernandez-Fernandez A., Martinez-Sanchez J., Sanchez-Munoz J.J., Garcia-Alberola A., Valdes-Chavarri M. Multiple interatrial electrical connection after heart transplantation. Pacing Clin Electrophysiol. 2012;35:e73–e75. doi: 10.1111/j.1540-8159.2011.03028.x. [DOI] [PubMed] [Google Scholar]
- 12.Minamiguchi H., Mizuno H., Masuda M., Sakata Y., Saito S., Nanto S., Sawa Y., Komuro I. Catheter ablation of focal atrial tachycardia originating from a donor heart after bicaval orthotopic heart transplantation guided by a noncontact mapping system. Int Heart J. 2012;53:146–148. doi: 10.1536/ihj.53.146. [DOI] [PubMed] [Google Scholar]
- 13.Elsik M., Teh A., Ling L.H., Virdee M., Parameshwar J., Fynn S.P., Kistler P.M. Supraventricular arrhythmias late after orthotopic cardiac transplantation: electrocardiographic and electrophysiological characterization and radiofrequency ablation. Europace. 2012;14:1498–1505. doi: 10.1093/europace/eus092. [DOI] [PubMed] [Google Scholar]
- 14.Makanjee B., Klein G.J., Derval N., Skanes A.C. An anterior ablation line is preferred for perimitral flutter after heart transplant. J Cardiovasc Electrophysiol. 2010;21:574–576. doi: 10.1111/j.1540-8167.2009.01657.x. [DOI] [PubMed] [Google Scholar]
- 15.Solheim E., Off M.K., Hoff P.I., Ohm O.J., Chen J. Electroanatomical mapping and radiofrequency catheter ablation of atrial tachycardia originating from the donor heart after orthotopic heart transplantation in a child. J Interv Card Electrophysiol. 2009;25:73–77. doi: 10.1007/s10840-008-9350-y. [DOI] [PubMed] [Google Scholar]