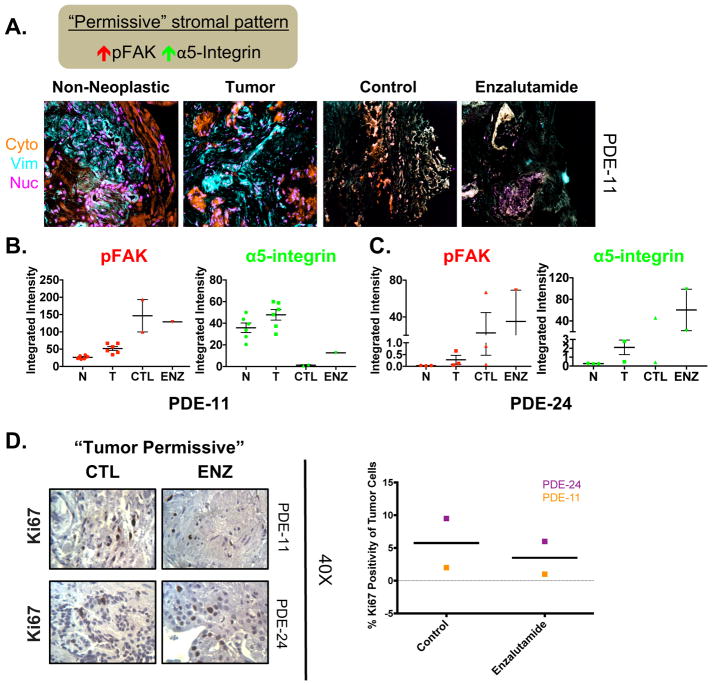
Figure 5. Initial “tumor permissive” stromal pattern in the PDE model indicates possible resistance to enzalutamide.
A. Top: Schematic explaining TME characteristics indicative of “permissive” stromal type. Bottom: Representative Simultaneous Multiplex Immunofluorescence (SMI) image of masks (i.e. cytokeratin, vimentin, and nucleus) in PDE of non-neoplastic at Day 0 (N), tumor at Day 0 (T), and tumor tissue treated with either vehicle (CTL) or Enzalutamide (ENZ) for 6 days. Example of a “tumor permissive” TME with desmoplastic traits displaying high pFAK and high active α-5-integrin in tumor tissue. B. Quantification of the markers assessed in stromal areas: phospho-focal adhesion kinase (pFAK) and active α5-integrin. C. Additional patient with a “tumor permissive” stromal pattern. D. Ki67 staining was performed to determine the amount of proliferation that occurred in order to evaluate the effect of drug treatment on PDE growth. Representative Ki67 images (40X magnification) and quantification are shown for vehicle and AR antagonist (Enzalutamide) treated PDE. n=2.