Abstract
Body condition scoring (BCS) is a simple, rapid, noninvasive tool used to assess body condition in animals. In this study, we developed and validated a diagram-based BCS for adult zebrafish (Danio rerio), a popular research model. After receiving 20 min of hands-on training regarding the scoring system, 5 people each rated 95 adult zebrafish. The fish then were euthanized and measured to establish body condition indices (BMI and the Fulton K factor). Both condition indices were highly correlated with fish width. Using correlation data and observed trends in fish width, we established expected BCS definitions. We validated the BCS definitions in 2 ways. First, we calculated the Pearson correlation coefficient between the average observed BCS and expected BCS; this statistic revealed very strong correlation between observed and expected BCS. In addition, we assessed the predictive power of BCS by using multinomial logistic regression and then applied the fitted model to evaluate the accuracy of the predictions (BCS compared with BMI, 85%; BCS compared with K factor, 61%). Finally, to determine the robustness of BCS to variation among raters, we calculated the intraclass correlation coefficient and demonstrated high interrater reliability. In conclusion, adult zebrafish BCS can be used to quickly identify animals with different body condition indices (thin to obese). In addition, the diagram-based chart is easy to use and implement accurately, with minimal training.
Abbreviation: BCS, body condition score
Visual methods for body condition scoring (BCS) have been used in multiple animal species as simple and rapid tools to assess health and welfare.3,8,14,17,18,20 In addition, BCS is used to identify humane endpoints in biomedical research.3,8,18 Most BCS methodologies are semiquantitative and use both visual assessment and palpation of anatomic sites to score body condition.8,18 However, palpation is impractical in some species, due to their size, environment, and behavior; therefore, in these cases, BCS is reliant solely on visual assessment.14,17 Veterinarians, research staff, and animal care staff use BCS, behavior, clinical presentation, and other parameters, such as weight, to access the health of research animals. Zebrafish (Danio rerio) have become a popular model organism for investigation of a wide variety of biomedical research disciplines, including the mechanisms responsible for congenital birth defects, cancer, cardiovascular disease, neurodegeneration, and obesity, among many others.1,2,4,5,9 Although knowledge regarding the developmental and disease processes in zebrafish has increased, little work has been done to establish best practices for zebrafish husbandry.
As the zebrafish model grows in popularity, the need to rapidly monitor the health and welfare of individual animals increases. In the aquaculture and fishery industries, body condition is used to monitor the health and welfare of fish populations but is often calculated according to physical measurements of weight and standard length (snout to caudal peduncle fin base).1,11,12,16 Zebrafish are much smaller than common aquacultured species, making handling for measurement stressful to the fish and impractical when working with large populations. Therefore, other methods of health assessment are required to provide rapid identification of fish in poor condition so that care staff can better evaluate and address their welfare.
The visual BCS for adult zebrafish is a simple tool that requires minimal training and provides a mechanism through which animal care staff and veterinarians can quickly identify animals that require further evaluation. In the current study, we developed and validated a visual body condition scoring system (score, 1 through 5) to assess fish according to established fishery and research fish body condition indices during routine husbandry health checks. We also determined the interobserver subjectivity of our visual assessment BCS technique after observers received minimal training. In addition, according to the BCS, we have implemented basic humane endpoints to maintain fish health and welfare in our facility. To our knowledge, a visual BCS with diagram-based training had not previously been developed for zebrafish. Our study likely will provide valuable data for zebrafish laboratories faced with the need for accurate colony health assessment on a large scale and defines criteria for humane endpoints. Moreover, this information provides a paradigm for a systematic approach to evaluation of body condition scoring in zebrafish, a technique that can be adopted broadly as new zebrafish experimental and health needs emerge.
Materials and Methods
Animals.
Zebrafish (EK strain) were obtained from the Shared Zebrafish Facility of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Rockville, MD). The EK is a wild-type line obtained in 1998 (Ekkwill Tropical Fish Farm, Gibsonton, FL) and maintained by NIH.10 A cohort of breeders were reared within the Aquatic Habitats Mass Embryo Production Systems by MBK Installations (Calverton, United Kingdom). The ratio of females to males was 60:40 in a total population of 3500 adults. Facility practice is to retire breeders after 6 mo of breeding every other week. A random subset of retired zebrafish was used for this study. Animal procedures were done with IACUC approval.
Husbandry.
The aquatic animal program at the Shared Zebrafish Facility meets all federal regulations and has been fully AAALAC-accredited since 1999. Zebrafish are housed in polycarbonate fish tanks on racks (Aquaneering, San Diego, CA). This facility uses a recirculating aquaculture system with mechanical filtration to 20 µm, biologic filtration sand filters, and UV sterilization (2 parallel units; 1950 W, 13 low-pressure lamps at 254 nm). Facility water-quality parameters are maintained within safe limits (Upper limit of NH3 range, 0.02 mg/L; upper limit of NO2 range, 10 mg/L; upper limit of NO3 range, 40 mg/L; temperature, 26 to 29.4 °C; pH, 6.7 to 7.3; conductivity, 920 to 1100 µS/cm; dissolved O2, 6.0 to 7.8 mg/L). Water changes range from 12% to 14% daily (supplemented with Instant Ocean Sea Salt [Blacksburg, VA]). A formulated dry pellet feed (Gemma Micro 300, Skretting Nutreco, Tooele, UT) was fed to adult fish once daily at a designated amount of approximately 3% body mass and directly proportional to the density of fish within the tank. Routine tankside health examinations of all fish were conducted and documented by an aquatics specialist twice daily. Zebrafish colonies are screened biannually for Pseudoloma neurophilia, Mycobacterium spp., Edwardsiella spp., Flavobacterium spp., Pleistophora spp., ectoparasites, and endoparasites by using an indirect sentinel program. At the time of the study, none of the listed pathogens was detected.
Visual BCS for adult zebrafish.
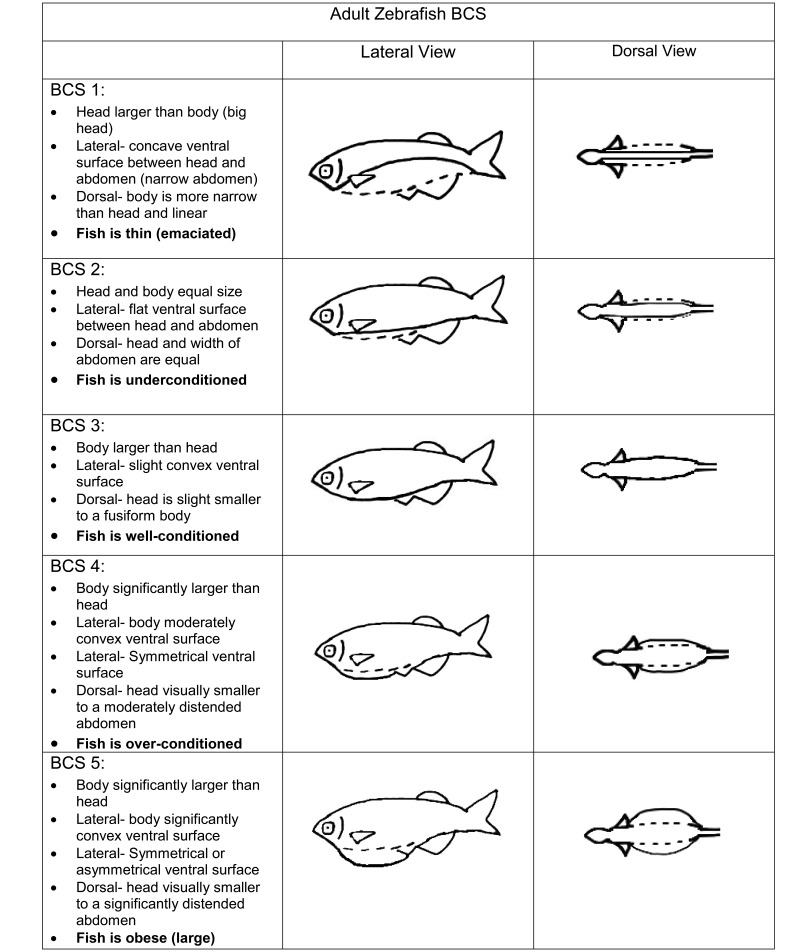
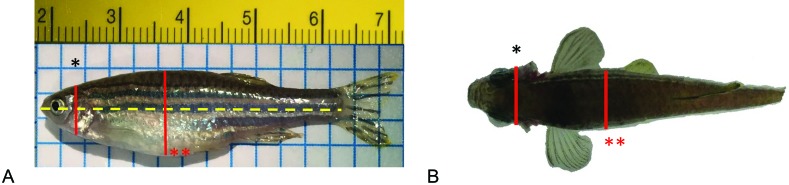
The visual BCS for adult zebrafish ranged from 1 to 5, with no subdivision (Figure 1). The BCS tool focuses on the size and shape of the abdomen as well as its relationship to the head to classify animals within the 5 categories (Figure 1). The 2 anatomic components assessed are the region of the head between the eye and operculum and the abdominal width (Figure 2). The landmarks of the abdomen vary slightly depending on the view; laterally, the abdominal region of interest is approximately halfway between the pectoral fin and anal fin, whereas dorsally the abdominal region of interest is approximately halfway from pectoral fin to dorsal fin. When using fins to identify regions, the cranial attachments of each fin are used as start and end points (Figure 2). Terms used to define BCS are based on standard welfare terms and physical characteristics of the abdomen.7
Figure 1.
Diagram-based chart and description of BCS, with supporting images (lateral and dorsal views).
Figure 2.
Targeted anatomic regions for BCS assessment.
Personnel training.
A total of 5 facility personnel were selected to participate as raters and comprised 2 veterinarians, 2 aquatic specialists, and 1 administrative staff member. The day before the visual inspection, a 20-min hands-on training session was given, and each person received the visual BCS chart. A 5-min verbal instruction covering BCS methodology and chart use was provided. The participants then had the opportunity to evaluate the BCS of random colony fish according to the chart and to ask questions. The day after completing training, each rater had 120 min to evaluate the BCS of the study animals. Each person evaluated the study animals individually and was blinded to the observations of other raters, and no evaluation periods overlapped.
Experimental procedure.
Prior to observations, study zebrafish were divided into same-sex groups. A total of 95 fish (48 male, 47 female) were collected randomly from the larger population. The selected animals were pooled, randomly single-housed in 1.8-L tanks, and placed on the recirculating system. Each tank was identified by a space location and a tank number (1 to 95). The following day, each team member received an evaluation sheet and a random distribution list of tanks, based on space location. Raters followed the space noted on their sheet to evaluate the BCS of the fish located in that space. Fish fasted during the observation period for a total of 24 h. The following day, all fish were euthanized individually in a 4 °C ice bath. Each fish was blotted dry and weighed to the nearest 0.001 g (Pioneer PA224, OHAUS, NJ). Each fish was photographed next to a ruler. Standard length and abdominal width measurements were obtained by 2 additional people using ImageJ software (version 1.51j8, NIH; Figure 2). All data was entered into an Excel spreadsheet (Microsoft, Redmond, WA). We calculated body condition indices by using 2 methods: BMI (weight [g] / standard length [mm]2) and the Fulton K factor (mass [g] / standard length [mm]3 × N. N is an integer to bring the value of K near 1.
Data analysis.
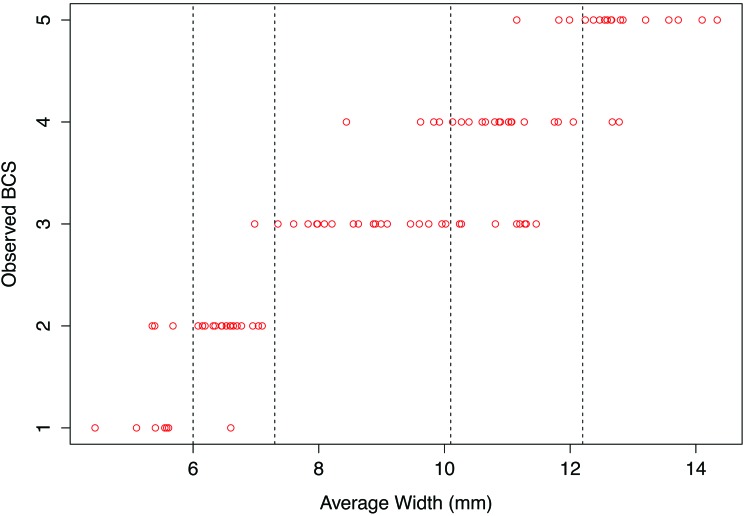
A composite BCS was calculated per rater by summing the lateral- and dorsal-view scores and dividing by 2 for each fish. The average BCS for all raters was calculated by adding the raters’ scores per fish and dividing by the number of raters. The average and composite BCS were rounded to the nearest whole number. We used the R program13 to perform statistical analyses. To correlate BCS with BMI and K factor indices, Pearson correlation coefficients were calculated between the 2 condition indices and measured width. On the basis of the correlation results and observable trends in the distribution of fish width, expected BCS categories were created. The robustness of the BCS category definitions was assessed in the following ways. First, we calculated a Pearson correlation coefficient between the average BCS and expected BCS according to our defined categories. Second, we fit multinomial logistic regression models separately between the expected BCS and BMI and between the BCS and K factor, to measure prediction accuracy. Finally, to determine interrater reliability, an intraclass correlation coefficient was calculated. The R package NNET19 was used to perform logistic regression, and the package IRR6 was used to calculate ICC.
Results
We assessed BMI in a total of 95 adult zebrafish (48 male, 47 female) for this study. Each reviewer had 120 min to evaluate BCS (mean, 71.8 min; median, 60.0 min; Table 1). Pearson correlation analysis was run to assess the linear association between BMI and K factors with width. Both condition indices were highly correlated with fish width (BMI: ρ = 0.975, P < 2.2 × 10–16; K factor: ρ = 0.898, P < 2.2 × 10–16). Using the correlation data and observed trends in fish width, we established the expected BCS definitions were established (Figure 3). Next, we validated our BCS definitions in 2 ways. First, we calculated a Pearson correlation coefficient between the average observed BCS and the expected BCS according to our defined categories and noted strong correlation between the 2 measures (ρ = 0.92, P < 2.2 × 10–16). Second, we assessed the predictive power of BCS by using multinomial logistic regression, which was run separately on BCS compared with BMI and BCS compared with K factor. We then used the fitted model to evaluate the accuracy of the predictions. For BCS compared with BMI, the predictive accuracy was 85%; for BCS compared with K factor, the predictive accuracy was 61%. Thus, the 2 metrics both confirmed that our expected BCS definitions were appropriate. Finally, to determine the robustness of BCS to variation between raters, we calculated the intraclass coefficient, a measure of interrater reliability. For our dataset, the intraclass coefficient was high (0.893, P = 5.26 × 10–154), indicating excellent agreement between raters.
Table 1.
Descriptive statistics
| Mean ± 1 SD | Range | |
| Length (mm) | 34.91 ± 4.28 | 25.32–45.80 |
| Width (mm) | 9.42 ± 2.55 | 4.44–14.34 |
| Weight (g) | 0.88 ± 0.45 | 0.23–2.15 |
| BMI (g/mm2) | 0.67 ± 0.22 | 0.27–1.22 |
| Fulton K factor (g/mm3 × 105) | 0.19 ± 0.05 | 0.83–2.96 |
Figure 3.
Distribution of average fish width compared with observed BCS. The vertical dotted lines correspond to boundaries delineating BCS zones.
Discussion
The data from this study support the conclusion that BCS can be used to accurately and quickly identify animals that have visibly different body condition indices (thin to obese). We developed the adult zebrafish BCS on the basis of 2 criteria: the ability to perform visual health assessments by tankside exam and common anatomic sites for white adipose tissue depots in adult fish.9 We developed a chart that included 2 images, a lateral and a dorsal view. In addition, we validated the zebrafish visual BCS to established fishery and research fish body condition indices (BMI and Fulton K factor).1,11,12,16 The selected condition indices are used to quickly estimate the energy or lipid content and use fish length–mass relationships.15 Both indices are positively correlated with width, the basis of the BCS scale of 1 to 5, and the Pearson correlation coefficient is highly statistically significant. The visual BCS derived from our observations does not correlate directly to adipose tissue compared with lean body mass; however, given the correlation, we infer that such a relationship may exist. In addition, we established the predictive accuracy of the BCS compared with BMI or K factor. BCS compared with BMI had a higher predictive accuracy and likely is more accurate for smaller fish. The K factor is often used with large aquaculture fish and therefore has limited use as a condition factor for zebrafish.
We surmise that, in practice, large-scale population-based body condition evaluations will use the lateral view most frequently; however, some tank configurations allow for an initial dorsal-view assessment of fish. When a more in-depth veterinary assessment of the BCS is required, we recommend using a composite score of both lateral and dorsal assessments, rounded to the nearest whole number. Finally, for this study, 5 people were sufficiently trained in using this chart in only 20 min, making it an easy tool to implement. The intraclass coefficient indicates an excellent agreement between raters, even with minimal training and rater experience. This time-efficient training illustrates that the diagram-based chart is easy to use and quick to implement accurately. Although we sampled similar numbers of male and female zebrafish, we did not assess potential sex-associated differences in BCS evaluation. We decided not to evaluate sex-associated effects in this study in light of the increased training that would be needed to differentiate between the sexes, which might have influenced the intraclass coefficient for BCS. Therefore, further studies evaluating the potential effects of zebrafish sex on BCS are needed.
With the popularity and utility of the zebrafish model, the need for well-developed health assessment tools that support more effective health and welfare monitoring are needed. BCS can be an important tool to assess both the population-based and individual health of adult zebrafish. Use of this tool will enable zebrafish research facilities to provide improved welfare monitoring and health concern response. In addition, this tool can help identify and eliminate unthrifty animals from the population, thus protecting the colony from potential vectors for disease and potentially reducing the disease burden overall. The BCS tool is an important mechanism through which facilities can incorporate the refinement of welfare by enabling investigators to increase the amount of information gathered regarding health and welfare. With this tool, we can better minimize pain and distress through rapid identification and thus enhance overall colony welfare.
In this study, the visual BCS did not correlate directly to adipose tissue compared with lean body mass; however, we think that the tool is effective for both population-based health assessment and individual animal welfare. The visual BCS for adult zebrafish offers a visual approximation of body condition without causing additional stress due to handling the fish. The BCS tool enables rapid body condition assessment by the husbandry staff, allowing them to perform a high throughput analysis of population-based health assessment without excessive labor and time costs. In addition, it is a valid and simple method for monitoring the health and welfare of zebrafish. Furthermore, the tool provides a mechanism for targeted veterinarian care on both individual animals and the colony at large. Changes in visual BCS in individual animals may be a welfare indicator requiring intervention. BCS scoring results might indicate underlying subclinical health concerns leading to wasting disease or identify tanks or cohorts that are not thriving and that might merit further diagnostic investigation. Further study to assess the BCS as an indicator of progressive illness or chronic disease is warranted. The integration of the BCS with other visual health assessments21 and incorporation of a standardized approach to zebrafish health and welfare7 will benefit both the model and facilities when evaluating health and welfare parameters.
References
- 1.Bolger T, Connolly PL. 1989. The selection of suitable indices for the measurement and analysis of fish condition. J Fish Biol 34:171–182. 10.1111/j.1095-8649.1989.tb03300.x. [DOI] [Google Scholar]
- 2.Bradford YM, Toro S, Ramachandran S, Ruzicka L, Howe DG, Eagle A, Kalita P, Martin R, Taylor Moxon SA, Schaper K, Westerfield M. 2017. Zebrafish models of human disease: gaining insight into human disease at ZFIN. ILAR J 58:4–16. 10.1093/ilar/ilw040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clingerman KJ, Summers L. 2012. Validation of a body condition scoring system in rhesus macaques (Macaca mulatta): inter- and intrarater variability. J Am Assoc Lab Anim Sci 51:31–36. [PMC free article] [PubMed] [Google Scholar]
- 4.Dooley K, Zon LI. 2000. Zebrafish: a model system for the study of human disease. Curr Opin Genet Dev 10:252–256. 10.1016/S0959-437X(00)00074-5. [DOI] [PubMed] [Google Scholar]
- 5.Fontana BD, Mezzomo NJ, Kalueff AV, Rosemberg DB. 2018. The developing utility of zebrafish models of neurological and neuropsychiatric disorders: a critical review. Exp Neurol 299:157–171. 10.1016/j.expneurol.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Gamer M, Lemon J, Fellows Puspendra Singh I. [Internet]. 2012. irr: various coefficients of interrater reliability and agreement. R foundation for statistical computing. [Cited 2 April 2018]. Available at: https://cran.r-project.org/web/packages/irr/index.html
- 7.Goodwin N, Karp NA, Blackledge S, Clark B, Keeble R, Kovacs C, Murray KN, Price M, Thompson P, Bussell J. 2016. Standardized welfare terms for the zebrafish community. Zebrafish 13 S1:S164–S168. 10.1089/zeb.2016.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hickman DL, Swan M. 2010. Use of a body condition score technique to assess health status in a rat model of polycystic kidney disease. J Am Assoc Lab Anim Sci 49:155–159. [PMC free article] [PubMed] [Google Scholar]
- 9.Imrie D, Sadler KC. 2010. White adipose tissue development in zebrafish is regulated by both developmental time and fish size. Dev Dyn 239:3013–3023. 10.1002/dvdy.22443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawson ND, Weinstein BM. 2002. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol 248:307–318. 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- 11.Oka T, Nishimura Y, Zang L, Hirano M, Shimada Y, Wang Z, Umemoto N, Kuroyanagi J, Nishimura N, Tanaka T. 2010. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol 10:1–13. 10.1186/1472-6793-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Priestley SM, Stevenson AE, Alexander LG. 2006. Growth rate and body condition in relation to group size in black widow tetras (Gymnocorymbus ternetzi) and common goldfish (Carassius auratus). J Nutr 136 7 Suppl:2078S–2080S. 10.1093/jn/136.7.2078S. [DOI] [PubMed] [Google Scholar]
- 13.R Core Team. [Internet]. 2015. R: a language and environment for statistical computing. [Cited 2 April 2018]. Available at: https://scholar.google.ca/citations?user=yvS1QUEAAAAJ&hl=en
- 14.Schiffmann C, Clauss M, Hoby S, Hatt J-M. 2017. Visual body condition scoring in zoo animals – composite, algorithm and overview approaches. J Zoo Aquar Res 5:1–10. 10.19227/jzar.v5i1.252 [DOI] [Google Scholar]
- 15.Schloesser RW, Fabrizio MC. 2017. Condition indices as surrogates of energy density and lipid content in juveniles of 3 fish species. Trans Am Fish Soc 146:1058–1069. 10.1080/00028487.2017.1324523. [DOI] [Google Scholar]
- 16.Siccardi AJ, Garris HW, Jones WT, Moseley DB, D'Abramo LR, Watts SA. 2009. Growth and survival of zebrafish (Danio rerio) fed different commercial and laboratory diets. Zebrafish 6:275–280. 10.1089/zeb.2008.0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomson JA, Burkholder D, Heithaus MR, Dill LM. 2009. Validation of a rapid visual-assessment technique for categorizing the body condition of green turtles (Chelonia mydas) in the field. Copeia 2009:251–255. 10.1643/CE-07-227. [DOI] [Google Scholar]
- 18.Ullman-Culleré MH, Foltz CJ. 1999. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab Anim Sci 49:319–323. [PubMed] [Google Scholar]
- 19.Venables WN, Ripley BD. 2002. Modern applied statistics with S, 4th ed New York (NY): Springer. [Google Scholar]
- 20.Vieira A, Brandão S, Monteiro A, Ajuda I, Stilwell G. 2015. Development and validation of a visual body condition scoring system for dairy goats with picture-based training. J Dairy Sci 98:6597–6608. 10.3168/jds.2015-9428. [DOI] [PubMed] [Google Scholar]
- 21.Wilson C, Dunford K, Nichols C, Callaway H, Hakkesteeg J, Wicks M. 2013. Body condition scoring for laboratory zebrafish. Animal technology and welfare : journal of the Institute of Animal Technology 12:1–7. [Google Scholar]