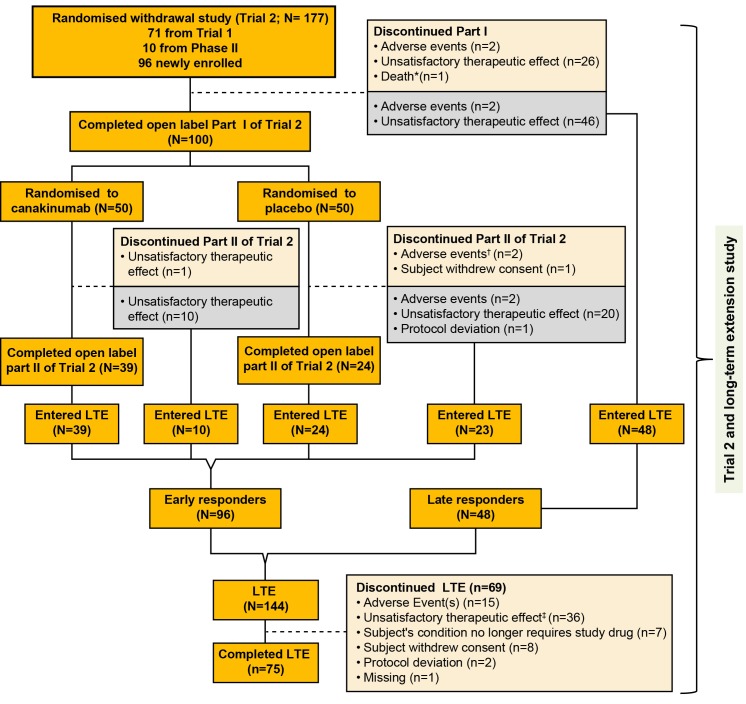
Figure 1.
Flow chart with patient disposition. *One death occurred during part I; patient died due to MAS. †A patient in the placebo group died due to MAS 2 days after discontinuing the part II phase due to MAS. ‡One patient died from disease progression 3 months after discontinuation from the long-term extension phase due to unsatisfactory therapeutic effect. The grey box represents the patients who discontinued the part I or part II of trial 2 and entered the long-term extension study. Patients who entered the LTE are divided into two subgroups: (1) early responders, defined as patients who had successfully completed the glucocorticoid tapering in part I of trial 2 as per protocol and who were randomised to the withdrawal part; (2) late responders, defined as patients who moved directly from the open-label part of trial 2 and who failed to taper glucocorticoids in part I. LTE, long-term extension; MAS, macrophage activation syndrome.