Abstract
The fascial system builds a three-dimensional continuum of soft, collagen-containing, loose and dense fibrous connective tissue that permeates the body and enables all body systems to operate in an integrated manner. Injuries to the fascial system cause a significant loss of performance in recreational exercise as well as high-performance sports, and could have a potential role in the development and perpetuation of musculoskeletal disorders, including lower back pain. Fascial tissues deserve more detailed attention in the field of sports medicine. A better understanding of their adaptation dynamics to mechanical loading as well as to biochemical conditions promises valuable improvements in terms of injury prevention, athletic performance and sports-related rehabilitation. This consensus statement reflects the state of knowledge regarding the role of fascial tissues in the discipline of sports medicine. It aims to (1) provide an overview of the contemporary state of knowledge regarding the fascial system from the microlevel (molecular and cellular responses) to the macrolevel (mechanical properties), (2) summarise the responses of the fascial system to altered loading (physical exercise), to injury and other physiological challenges including ageing, (3) outline the methods available to study the fascial system, and (4) highlight the contemporary view of interventions that target fascial tissue in sport and exercise medicine. Advancing this field will require a coordinated effort of researchers and clinicians combining mechanobiology, exercise physiology and improved assessment technologies.
Keywords: injury, consensus statement, tendon, soft tissue
Terminology and definitions
The term fascia was originally used to describe a sheet or band of soft connective tissue that attaches, surrounds and separates internal organs and skeletal muscles. Advancing research on the physiological and pathophysiological behaviours of a range of connective tissues has revealed that this definition is too restrictive. Understanding of mechanical aspects of connective tissue function depends on consideration of a host of interconnected and interwoven connective tissues beyond these sheets or bands, and there is enormous potential gain from understanding the convergence of biology underpinning adaptation, function and pathology.
The fascial system includes adipose tissue, adventitia, neurovascular sheaths, aponeuroses, deep and superficial fasciae, dermis, epineurium, joint capsules, ligaments, membranes, meninges, myofascial expansions, periostea, retinacula, septa, tendons (including endotendon/peritendon/epitendon/paratendon), visceral fasciae, and all the intramuscular and intermuscular connective tissues, including endomysium/perimysium/epimysium.1
With its diverse components, the fascial system builds a three-dimensional continuum of soft, collagen-containing, loose and dense fibrous connective tissue that permeates the body and enables all body systems to operate in an integrated manner (figure 1).1 In contrast, the morphological/histological definition describes fascia as ‘a sheet, or any other dissectible aggregations of connective tissue that forms beneath the skin to attach, enclose, and separate muscles and other internal organs’.1 The proposed terminology distinguishing the terms ‘fascia’ and ‘fascial system’ allows for the precise identification of individual structures as well as grouping them for functional purposes.
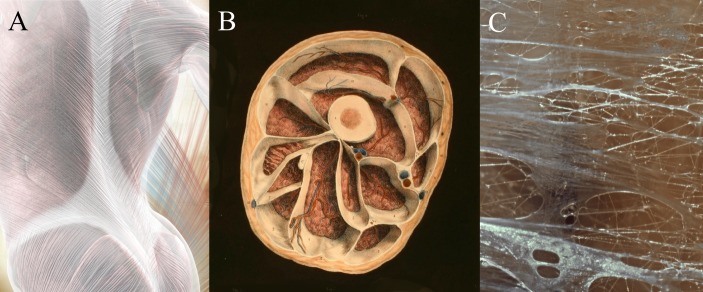
Figure 1.
Components of the fascial system. The fascial system includes large aponeuroses like the first layer of the thoracolumbar fascia (A), but also a myriad of enveloping containers around and within skeletal muscles (B) and most other organs of the body. The internal structure of fascial tissues is dominated by collagen fibres which are embedded in a semiliquid ground substance (C). Images with friendly permission from fascialnet.com (A) and thomas-stephan.com (C).
Consensus meeting
The Second International CONNECT Conference was held at the University of Ulm, Germany, on 16–19 March 2017, as part of a conference series aimed at fostering scientific progress towards a better understanding and treatment of fascial tissues in sports medicine. After the conference, a meeting was held with conference speakers and other field-related experts to discuss and find consensus regarding the role of fascial tissue in the field of sports medicine.
Injuries to a variety of fascial tissues cause a significant loss of performance in sports2 and have a potential role in the development and perpetuation of musculoskeletal disorders, including lower back pain.3 A major goal of clinicians is to return athletes and patients to activity, training and competition after injury.
This consensus statement reflects the current state of knowledge regarding the role of fascial tissues in the discipline of sports medicine and will be updated as part of a consensus meeting during the CONNECT conference. This paper aims to summarise the contemporary state of knowledge regarding the fascial system from the microlevel (molecular and cellular responses) to the macrolevel (mechanical properties), and the responses of the fascial system to altered loading (physical exercise), to injury and other physiological challenges including ageing, methods available to study the fascial system, and the contemporary view of interventions that target fascial tissue in sports medicine. This document was developed for scientists and clinicians to highlight common traps and truths of fascial tissue screening and imaging techniques and intervention methods, and to present a multidisciplinary perspective of future research in the field.
Molecular adaptation of fascial tissues: effects of physical exercise, ageing, sex hormones and inflammation
Molecular crosstalk between extracellular matrix (ECM) molecules and cellular components is an important determinant of fascial tissue physiology and pathophysiology. A molecular chain, characterised by high functional and structural plasticity and bidirectional molecular interactions, connects the cellular cytoskeleton to the ECM (figure 2). Small functional and structural alterations in the ECM result in complex cellular adaptation processes and, vice versa, changes in cell function and structure leading to ECM adaptation.4 Therefore, fascial tissue homeostasis is the result of a complex interplay and dynamic crosstalk between cellular components and the ECM. Especially under dynamic conditions such as growth and regeneration, strong alterations of the local ECM microenvironments are necessary to allow cellular adaptation and rebuilding of fascial tissues. All factors influencing cell or ECM behaviour can result in changes in the structure and homeostasis of tissues and organs.
Figure 2.
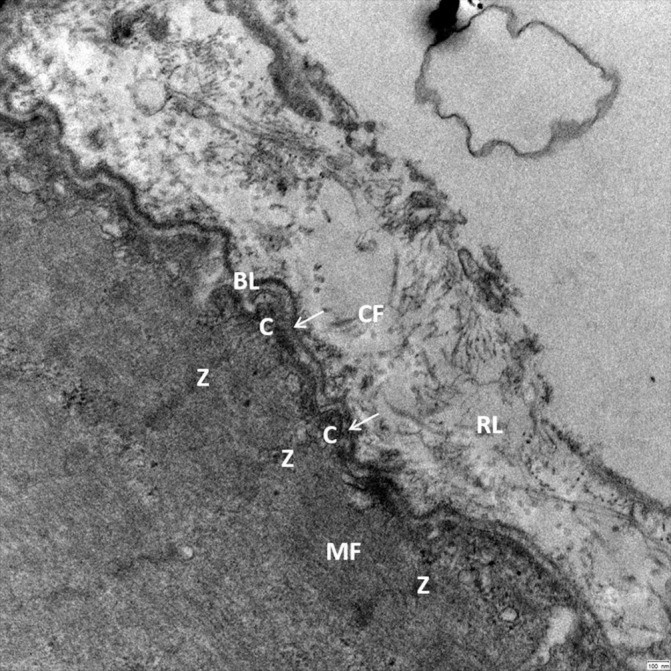
Transmission electron microscopy reveals the close cell–ECM interaction in human skeletal muscle (musculus vastus lateralis, 25 000× magnification) allowing a bidirectional cell–ECM interaction. Myofilaments (MF) are connected by Z-lines (Z) and costameres (C) to the adjacent basal lamina (BL) and the surrounding reticular lamina (RL). Crossbridging structures (arrows) connect the Z-lines and costameres to the dense part of the basal lamina. The reticular lamina is structured by a network of collagen fibrils (CF) and additional ECM molecules, which have a close connection to the basal lamina allowing bidirectional transmission of mechanical forces. ECM, extracellular matrix.
The ECM also works as a molecular store, catching and releasing biologically active molecules to regulate tissue and organ function, growth and regeneration. Molecules stored in the ECM network can be cleaved to release biologically active cleavage products.5 Mechanical stress can induce the release and activation of ECM-stored molecules, inducing the cleavage products of collagen XVIII and other basement membrane components. It has been shown that endostatin (the 20 kDa C-terminal fragment of collagen XVIII) can modulate vascular growth and function.6–8 In addition, changes in the ECM by ageing or physical exercise may be involved in triggering systemic effects via excreted circulatory molecules, such as the exercise-responsive myokine irisin,9 which has been proposed to increase energy expenditure in mice and humans.
In fascial tissues such as tendons, acute and chronic loading stimulates collagen remodelling.10 As the exercise-induced increase in collagen synthesis is lower in women than in men, and as injury frequency and the expression of oestrogen receptors in human fascial tissue are sex-dependent, oestrogens may play an important regulatory role in ECM remodelling.11–13 The effects of oestrogens on collagen synthesis appear to differ between rest and response to exercise. While oestrogen replacement in elderly, postmenopausal women impairs collagen synthesis in response to exercise, oestrogen has a stimulating effect on collagen synthesis at rest.14 Oral contraceptives, on the other hand, have an overall depressing effect on collagen synthesis.15
Physiological ageing is a highly individual process characterised by a progressive degeneration of tissues and organ systems. Age-related alterations in fascial tissues include densification (alterations of loose connective tissue) and fibrosis (alterations of collagen fibrous bundles).16 Functionally, these pathological changes can modify the mechanical properties of fascial tissues and skeletal muscle, thereby contributing to pain-related and age-related reductions in muscle force or range of motion, which cannot be solely explained by the loss of muscle mass.17 ECM structural, biochemical, cellular and functional changes occur during ageing.18 Interestingly, ageing is characterised by chronic, low-grade inflammation—the so-called inflammaging.19 As the ECM is the main site of inflammatory responses taking place in tissues, it is not surprising that the ECM can interact with immune cells to change their function, which is important for growth and regeneration of tissues. Leucocyte extravasation depends on cleavage of the basal membrane by locally released proteases. Tenascin and osteopontin are examples of ECM molecules important for the regulation of the local immune response.20 21 In addition, ECM plays an important role as a barrier to transmigration of immune cells in and out of the tissue. Although early inflammation after tissue damage due to physical exercise or injury is crucial for tissue remodelling and adaptation,22 23 stem cell activity and collagen synthesis may be inhibited by the chronic intake of non-steroidal anti-inflammatory drugs prior to exercise.24 25 However, limiting the magnitude of inflammation might be beneficial for tissue regeneration and gains in muscle mass and strength, depending on the nature of the injury,26 and in elderly people.27
Outlook and perspectives for future research: insights into the structure–function relationship of the ECM, especially in ageing and injured fascial tissues and skeletal muscle, are highly relevant for maintaining musculoskeletal function in the elderly during daily life and exercise and for prevention of exercise-related overuse injuries in athletes. While a body of literature exists on metabolic activity and ECM remodelling in human tendons in response to exercise, much less is known and more research is needed to investigate the molecular response of other fascial tissues (such as intramuscular fascial tissue) to altered loading and ageing.
Myofascial force transmission
Conventionally, skeletal muscles have been considered as primarily transmitting force to their osseous insertions through the myotendinous junction.28 However, in situ experiments in animals and imaging studies in humans have shown that intermuscular and extramuscular fascial tissues also provide a pathway for force transmission.29–33 Although the magnitude of non-myotendinous force transmission under in vivo conditions is disputed,34 35 the contribution of these pathways is thought to be dependent, in part, on the mechanical properties of myofascial tissue linkages.36 Myofascial tissue that is stiffer or more compliant than normal has been shown to influence the magnitude of intermuscular force transmission and, arguably, may have a significant effect on muscle mechanics.37–39 The mechanical properties of fascial tissues can be modified by several factors, which, inter alia, include a change in fluid content, crosslinks and molecular organisation and content of specific ECM molecules, and the contractile activity of myofibroblast cells.40 41 Changes can also be a consequence of muscle injury,42 disease,43 surgical treatment37 or ageing (figure 3).44
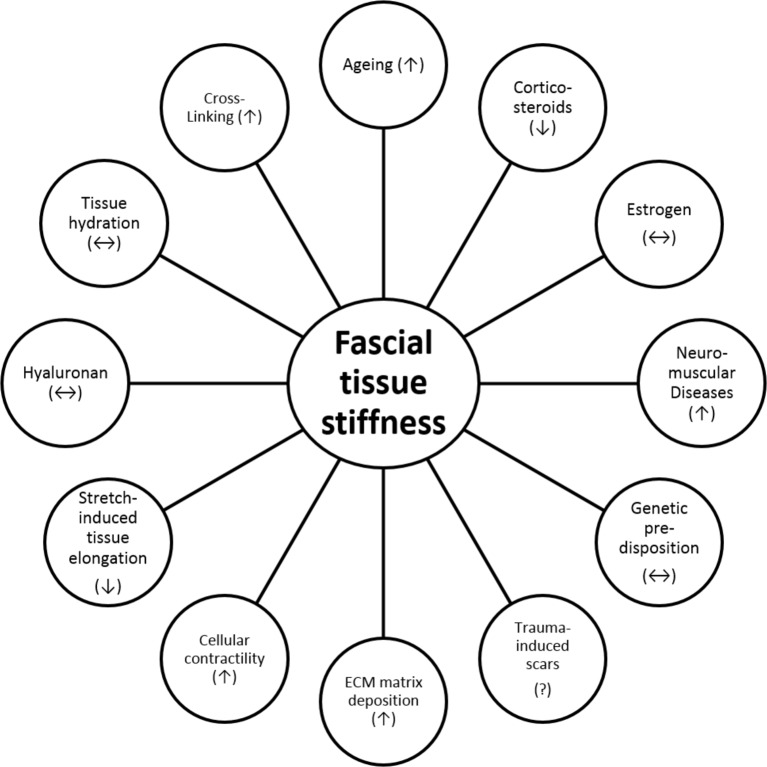
Figure 3.
Factors influencing the mechanical stiffness of fascial tissues and their hypothesised impact. Up arrows symbolise a positive effect (eg, increased cellular contractility increases stiffness), down arrows symbolise a negative effect (eg, increased use of corticosteroids decreases stiffness) and double arrows symbolise an ambiguous association (eg, hyaluronan decreases stiffness if mobilised by mechanical stimuli, but leads to increased stiffness if no stimuli are applied). ECM, extracellular matrix.
As fascial tissues connect skeletal muscles, creating a multidirectional network of myofascial continuity,45 altered local forces (eg, by muscular contraction) might also affect the mechanics of adjacent tissues. In fact, a plethora of cadaveric and animal studies have demonstrated substantial mutual interactions between neighbouring muscles arranged serially in slings (eg, latissimus muscle and gluteus maximus muscle)46 and parallel to each other (eg, lower limb synergists).47 For example, when seen from a fascial perspective, the knee-joint capsule is influenced by directly inserting tendons and by more distant structures such as the gluteus maximus or the tensor fasciae latae and their connecting fasciae.48 However, it remains to be further elucidated how such findings translate into human in vivo conditions.
Although scarce, initial in vivo evidence points towards a significant role of myofascial force transmission for the locomotor system. Available data point towards the existence of (1) remote exercise effects and (2) non-local symptom manifestations in musculoskeletal disorders, both of which might be of relevance in athletic and therapeutic settings. It has been shown that stretching of the lower limb increases the range of motion of the cervical spine, and patients with sacroiliac pain display hyperactivity of the gluteus maximus and the contralateral latissimus muscle.49–51 Because the involved body regions are connected via myofascial chains, myofascial force transmission might be the cause of the observations. Besides interactions between muscles arranged in series, significant amounts of force have been shown to be transmitted in vivo between muscles located parallel to each other; electrical stimulation of the gastrocnemius muscle leads to a simultaneous displacement of the soleus muscle.30 This intralimb myofascial force transmission may be of relevance in diseases such as cerebral palsy.38
Outlook and perspectives for future research: Although the basic mechanisms of myofascial force transmission have been studied, there is a need to discern the influence of variables, such as age, sex, temperature and level of physical activity, within healthy physiological and pathological settings. Furthermore, despite convincing in vitro evidence for the existence of myofascial force transmission, its relative contribution to the occurrence of remote exercise effects under in vivo conditions has to be further elucidated. Besides mechanical interactions between adjacent tissues, non-local changes of stiffness or flexibility may also (at least partly) stem from neural adaptations, for example, a systemic reduction of stretch tolerance.
Injury of fascial tissues: cellular and mechanical responses to damage
Excessive or prolonged loading or direct trauma to fascial tissues initiates micro and macro changes necessary for tissue repair. These effects may also contribute to pathological changes that modify tissue function and mechanics, leading to compromised function of the healthy tissue. Effects may become systemic, and thus not limited to the injured/loaded tissues.
Following an acute injury from overload or anoxia in fascial tissues, the immune response aims to phagocytose injured cells. An acute inflammatory response is typically short-lived and reversible and involves the release of a range of molecules, including proinflammatory cytokines from injured cells and macrophages, along with other substances (eg, bradykinin, substance P and proteases) that sensitise nociceptive afferents52 and promote immune cell infiltration. If loading is prolonged or repetitive, persistent inflammation may develop,53 54 leading to the prolonged presence of macrophages and cytotoxic levels of cytokines in and around tissues, ultimately resulting in ongoing tissue damage. Some tissue cytokines (eg, interleukin-1β, tumour necrosis factor (TNF) and transforming growth factor beta (TGFβ-1)) are fibrogenic cytokines that can promote fibrosis via excessive fibroblast proliferation and collagen matrix deposition.55
Overproduction of cytokines also maintains sensitisation of nociceptive afferents—a change that would increase production and release of substance P (a known nociceptor neuropeptide). Recent studies show that substance P can stimulate TGFβ-1 production by tendon fibroblasts, and that both substance P and TGFβ-1 can induce fibrogenic processes independently of each other.56
Taken together, these findings suggest that both neurogenic processes (nerves are the primary source of substance P) and loading/repair processes (TGFβ-1 is produced by fibroblasts in response to mechanical loading and during repair) can contribute to increased collagen in fascial tissues. Fibrosis (eg, collagen deposition) around the tendon, nerve and myofascial tissues influences dynamic biomechanical properties secondary to tissue adherence and can tether structures to each other or induce chronic compression.57 Increased collagenous tissues surrounding the nerves can tether the nerves and also enhance pain behaviours.58 Furthermore, inflammatory cytokines can ‘spill over’ into the bloodstream, leading to widespread secondary tissue damage and central nociceptor wind-up.53 59 Circulating TNF is elevated in chronic lower back pain,60 and recent data highlight a relationship between elevated TNF and greater risk for progression to chronic pain in some individuals61 and in animal models of overuse.59
Muscles also undergo changes in muscle fibre composition, adiposity and fibrosis in response to injury to related structures (eg, injury to an intervertebral disc) even in the absence of muscle trauma (figure 4). These changes closely resemble those identified for direct muscle trauma, such as supraspinatus tendon lesion,62 although with some differences (eg, differences in the distribution of infiltrating fat). After an injury to an intervertebral disc, deep back muscles undergo rapid atrophy,63 64 most likely mediated by neural changes such as reflex inhibition.65 This is followed by changes in muscle fibre composition (slow-to-fast muscle fibre transition), fibrosis and fatty infiltration associated with increased production of proinflammatory cytokines (eg, TNF).66 Increased cytokine expression was first identified from an mRNA analysis of the muscle, but with an unclear origin. Recent work suggests this is mediated by an increased proportion of proinflammatory macrophages,67 hypothesised to result from altered metabolic profiles of the muscle as a consequence of transition to more fast (fatigable) muscle fibres.68 Adipose tissue is a potential source of proinflammatory cytokines and has been implicated in a range of musculoskeletal conditions, including osteoarthritis.69 Regardless of the underlying mechanism, fibrotic changes in the muscle have a substantial potential impact on tissue dynamics and force generation capacity.
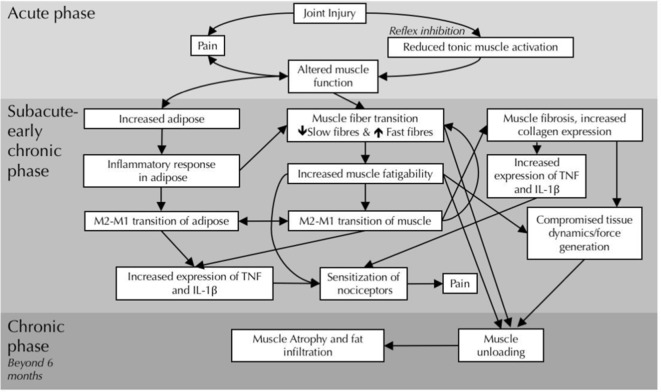
Figure 4.
Proposed timeline and mechanisms for fascial, adipose and muscle changes in the multifidus muscle after intervertebral disc lesion. Three phases, acute (top), subacute-early chronic (middle) and chronic (bottom), are characterised by different structural and inflammatory changes. IL-1β, interleukin-1β; TNF, tumour necrosis factor.
Exercise, physical modalities and pharmacological interventions have all been shown to reduce the inflammatory processes associated with fascial tissue injury and fibrosis. For example, early treatment with anti-inflammatory drugs can prevent/reverse pain behaviours induced by TNF signalling and reduce downstream collagen production in animal models.70 Stretching of fascial tissues can promote resolution of inflammation both in vivo and in vitro,71 and manual therapy can prevent overuse-induced fibrosis in several fascial tissues.72 In terms of muscle changes, resistance exercise is necessary to reverse fatty changes (and perhaps fibrosis) in chronic conditions,73 whereas gentle muscle activation is sufficient to reverse early muscle atrophy,74 and whole body exercise can prevent inflammatory changes in back muscles that follow intervertebral disc injuries.75
Outlook and perspectives for future research:Future research is needed to gain a deeper understanding of the mechanisms underlying the impact of treatments on fibrosis and fatty changes in fascial tissues. Although there is evidence that exercise, physical therapies or pharmacological approaches can impact inflammatory processes, and reduce consequences, further work is required to understand how best to tailor interventions based on the time-course of pathology and type of exercise, or whether there is additional benefit from combined treatments.
Imaging and non-imaging tools for diagnosis and assessment
Pathological changes in the mechanical properties of fascial tissues have been hypothesised to play an essential role in musculoskeletal disorders such as chronic pain conditions and overuse injuries.76 As a result, considerable demand for diagnostic methods examining fascial tissue function has arisen. In basic research, an oft-used approach is to study molecular and mechanical changes in myofibroblasts and other biomarkers via needle biopsy and subsequent immunohistochemistry.77
To evaluate the effects of treatment and exercise in clinical settings, a series of methods are available (table 1). Changes in water content can be analysed via bioimpedance assessment,78 but there are no data on reliability and validity of measurements in smaller body regions. Manual palpation represents a cost-neutral and widely used screening method aimed at assessing viscoelastic properties (eg, stiffness); however, similarly, its reliability is limited.48 79 80 81 However, the approach is based on a number of assumptions, and available devices often lack a thorough proof of validity.77 82 Moreover, no tissue-specific conclusions can be drawn due to the black-box character of the measurements.83 Imaging methods such as ultrasound or elastography, in contrast, are promising tools for explicitly quantifying the mechanical properties of fascial tissues under in vivo conditions.84
Table 1.
Currently used diagnostic methods to examine fascial tissue structure and function
| Method | Assessment target | Advantages | Disadvantages | References |
| Biopsy | Histological properties including molecular analysis. | Permits analysis of tissue damage, infiltration of inflammatory cells, cytokines and others. | Invasiveness. | 66 75 77 |
| Bioimpedance | Hydration changes. | High sensitivity. | Lacking data on reliability and validity for smaller regions. | 78 |
| Manual palpation | Stiffness, elasticity and shearing mobility of tissue. | Cost-effectiveness. Psychosocial factors. |
Limited reliability. | 79 80 82 |
| Indentometry | Stiffness and elasticity. | Established reproducibility. | Limited depth. | ) 81 83–85 |
| Ultrasound (US) imaging | Thickness of layers, tendon elongation. | Permits diagnosis of a fibrotic thickening (eg, of a particular endomysium) or of tendon strain response during loading. | Difficulty in standardising the exact viewing angle. | 86 88 |
| US with correlation software | Relative shearing motion of adjacent layers. | Permits diagnosis of adhesive tissue connections, such as in chronic low back pain. | Lacking standards for selection of regions of interest. | 89 |
| Compression-based US elastography | Stiffness. | Measurements possible at further depth than, for example, with indentometry. | Lack of standardisation. Frequent appearance of artefacts. |
87 |
| Shear-wave US elastography | Stiffness. | Enhancement by propagation analysis permits morphological analysis. | Lack of standardisation. | 90 91 |
| B-mode ultrasonography | Tendon structure and mechanical/material properties. |
|
|
90 96–98 103 |
Producing a distortion of the measured tissue (eg, through compression or shear waves), elastography provides ultrasound images reflecting the relative hardness of the targeted area. Recently, the technique has been increasingly applied in musculoskeletal research. However, the existence of several different methods, lack of standardisation and frequent appearance of artefacts during measurements threaten the validity of achieved results.85 Without the use of elastography, the conventional ultrasound image can be reliably used to display and measure the morphology of fascial tissues, such as myofascial tissues, ligaments and tendons.86 Some initial studies have, moreover, attempted to quantify relative movement (eg, sliding of fascial layers and shear strain) using cross-correlation calculations.87
Despite some initial applications to myofascial tissues, most data on ultrasound imaging are available for tendon measurements (figure 5). In the late 1990s, advancements made in the application of B-mode ultrasonography allowed quantification of the tensile deformation of human tendons, in vivo, based on tracking of anatomical features in the tendon when pulled on by the force exerted in the in-series muscle during static contraction.88 Unfortunately, the in vivo stiffness and Young’s modulus results often disagree with findings from in vitro material tests, when forces and elongations are precisely controlled and measured. Errors are likely being caused by in vivo measurement simplifications in the quantification of both tendon deformation and the loading applied during the static muscle contraction. The former includes simplifications regarding the tendon’s resting length, line of pull and uniformity in material properties. The latter includes simplifications regarding the effect of loading on tendon moment arm length, the effect of antagonist muscle coactivation and the uniformity in tendon cross-sectional area. Most of these simplifications can be avoided by appropriate measurements to quantify the neglected effects. In addition, recent developments in ultrasound shear-wave propagation89 and speckle tracking90 have the potential to substantially improve experimental accuracy and physiological relevance of in vivo findings.
Figure 5.
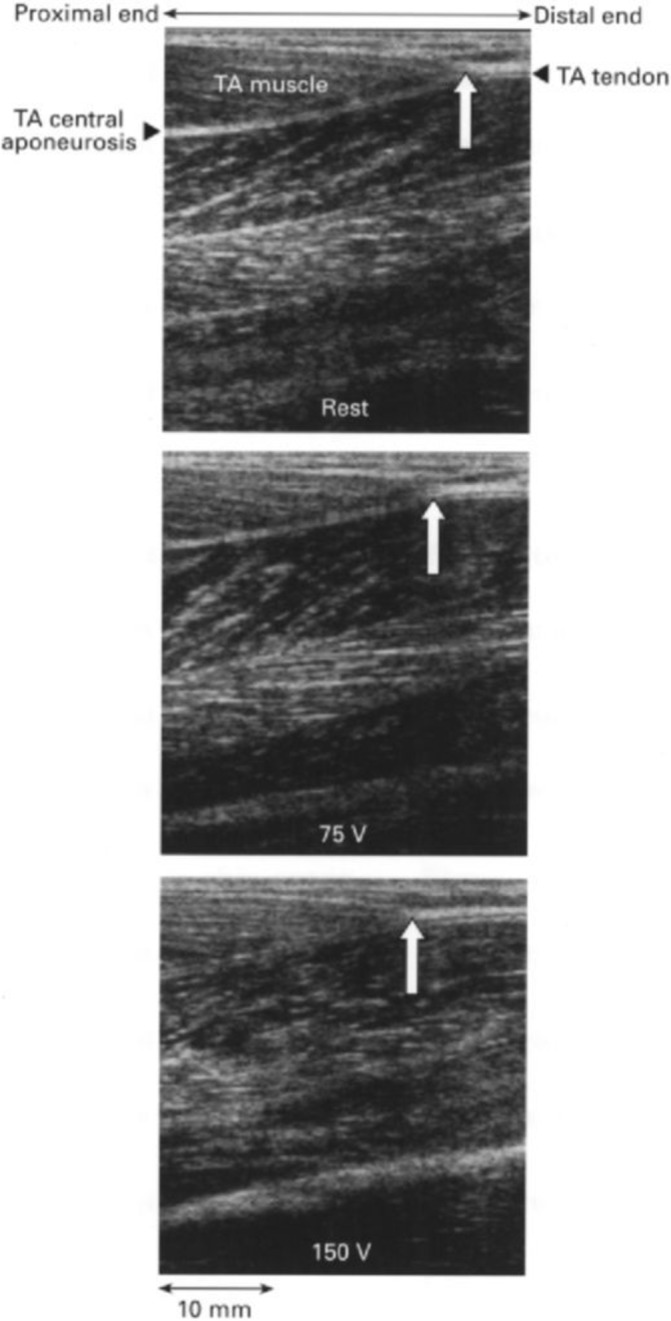
Tendon displacement measured by B-mode ultrasound. Sonographic images of the human tibialis anterior (TA) muscle at rest (top) and in response to electrical stimulation at 75 V (middle) and 150 V (bottom). The white arrow indicates the TA tendon origin. Notice the proximal shift of the TA tendon origin on electrical stimulation.88
In contrast to static muscle contraction tests aimed at assessing human tendon stiffness and Young’s modulus, scanning during dynamic activities has typically been applied to document tendon deformations directly, through morphometric analysis on scans,90 91 or indirectly, through ultrasound propagation speed analysis,92 93 to investigate the interaction between tendon and muscle in the studied task. These experimental approaches are relatively immune to problems caused by erroneous quantification of tendon forces; however, appropriate measurements need to be taken to validate the assumption that the usual practice of tracking a single tendon anatomical point, or a tendon region limited by the size of the scanning probe, can give a representative picture for the entire tendon.
Outlook and perspectives for future research: In view of the current diagnostic methods’ limitations, further research investigating the measurement properties (eg, validity) is warranted to provide evidence-based recommendations. Hence, within the clinical assessment of mechanical soft-tissue properties, collected data should be interpreted with caution, and, as long as no clear gold standards exist, a combination of methods seems advisable instead of focusing exclusively on one technique. Ultrasound-based assessments of tendon deformability on loading have grown in popularity but can provide erroneous conclusions due to several invalid assumptions and approximations typically made to simplify the experimental protocol. Most of these errors can be eliminated by appropriate measurements.
Mechanobiology of fascial tissues: effects of exercise and disuse
The main principles of the above ultrasound-based methodology have been implemented in numerous studies over the last 20 years to study the adaptability of human tendons to exercise and disuse.94 95 The findings convincingly show that human tendons respond to the application of chronic overloading by increasing their stiffness and to chronic unloading by decreasing their stiffness. The mechanisms underpinning these adaptations include changes in tendon size and changes in Young’s modulus. One common finding among studies is that tendon adaptations occur quickly, within weeks of mechanical loading/unloading application.96 97 Importantly, however, some studies report adaptations in tendon size but not tendon material,98 and others in tendon material but not size,96 while some report adaptations in both tendon size and material.99
To study human tendon mechanobiology and explore the basis of the above distinct adaptability features, both cross-sectional and longitudinal experimental designs have often been adopted. Cross-sectional designs have been used for the following purposes: (1) to compare tendons subjected to different habitual loads due to their specific anatomical location,100 (2) to compare tendons between limbs with muscle strength asymmetry,98 (3) to compare tendons in humans with different body mass but similar habitual activities95 and (4) to compare tendons in athletes with those in sedentary individuals.99 Study designs (1), (2) and (3) support the notion that adjustments in tendon stiffness to accommodate changes in physiological loading are accomplished by adding or removing tendon material rather than altering Young’s modulus of the tendon. Importantly, the addition or removal of tendon material does not seem to always occur uniformly along the tendon, but in some regions only, which can go undetected unless the whole tendon is examined.101 In contrast to study designs (1), (2) and (3), findings from study design (4) show that improvements in Young’s modulus of the tendon may occur and account fully for, or contribute to, the increased tendon stiffness in response to loading. Interestingly, exercise-training intervention studies also report improvements in Young’s modulus of the tendon.94–96 In combination, these findings indicate that stiffening of the tendon through alteration of its material requires ‘supra-physiological’ loading features (eg, in terms of loading magnitude, frequency and/or duration). Once this rapid adaptation occurs and the exercise becomes a habitual daily activity, alterations in tendon size might mediate any further changes in tendon stiffness.
Outlook and perspectives for future research: Combining ultrasonography with dynamometry methods has now made it possible to assess in vivo human tendon plasticity under conditions of altered mechanical loading. Two important questions warrant further research. (1) What is the mechanism underpinning regional differences in tendon adaptability in terms of tendon size? Possibilities worth investigating include differences in local stress, local Young’s modulus, local blood flow and mechanotransduction sensitivity. Finite element modelling of the tendon may be an appropriate avenue to examine the first two possibilities. (2) What is the limiting factor in tendon plasticity to exercise? An intuitive answer is that the magnitude and time-course of tendon plasticity are merely determined by how much and how fast the in-series muscle force increases as the muscle adapts to the chronically increased load, but confirming this requires systematic research.
Interventions for fascial tissue pathologies in sports medicine
Fascial tissue dysfunction in the field of sports medicine is rarely treated surgically. Anti-inflammatory drugs are used for sports-related overuse pathologies; however, they may impair regeneration and diminish tissue adaptation.24 25 Gyrase-inhibiting antibiotics often contribute to an increased likelihood of tendon injuries in sports.102 In addition, injections of platelet-rich plasma seem to be successful in some cases of tendinopathy, although efficacy remains inconclusive.67 103 Moderate evidence exists on the value of shockwave therapy and eccentric loading in tendon healing.104 105 Similarly, foam rolling (tool-assisted massage of myofascial tissues) seems to improve short-term flexibility and recovery from muscle soreness75 106 107 and decrease latent trigger point sensitivity.103 Nevertheless, the physiological mechanisms of these reported effects remain unclear, although initial evidence suggests increases in arterial perfusion, enhanced fascial layer sliding and modified corticospinal excitability following treatment108 109 (F Krause et al, submitted, 2018). Finally, manual therapies, such as massage, osteopathy or Rolfing (a massage technique based on achieving symmetrical alignment of the body), are frequently used to improve fascial tissue regeneration or athletic performance, although their efficacy still remains to be validated.110 111
Outlook and perspectives for future research: Hopefully, current and future improvements in assessment methodologies will generate more conclusive research regarding which treatment modalities are most promising for specific conditions. While commercial and other interests often favour the promotion of premature positive conclusions about specific fascia-related treatments, strict application of scientific rigour is essential for the development of this promising field.
Footnotes
RS and PWH contributed equally.
Funding: PWH is supported by a research fellowship from the National Health and Medical Research Council of Australia (NHMRC; APP1102905). Partial funding was provided by the Ida P Rolf Research Foundation.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Correction notice: This article has been corrected since it published Online First. The title has been amended.
Presented at: 2018 consensus statement from the Second International CONNECT Conference, Ulm, Germany.
References
- 1. Adstrum S, Hedley G, Schleip R, et al. . Defining the fascial system. J Bodyw Mov Ther 2017;21:173–7. 10.1016/j.jbmt.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 2. Ljungqvist A, Schwellnus MP, Bachl N, et al. . International Olympic committee consensus statement: molecular basis of connective tissue and muscle injuries in sport. Clin Sports Med 2008;27:231–9. 10.1016/j.csm.2007.10.007 [DOI] [PubMed] [Google Scholar]
- 3. Wilke J, Schleip R, Klingler W, et al. . The lumbodorsal fascia as a potential source of low back pain: a narrative review. Biomed Res Int 2017;2017:1–6. 10.1155/2017/5349620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen B, Ji B, Gao H. Modeling active mechanosensing in cell-matrix interactions. Annu Rev Biophys 2015;44:1–32. 10.1146/annurev-biophys-051013-023102 [DOI] [PubMed] [Google Scholar]
- 5. Suhr F, Brixius K, Bloch W. Angiogenic and vascular modulation by extracellular matrix cleavage products. Curr Pharm Des 2009;15:389–410. 10.2174/138161209787315756 [DOI] [PubMed] [Google Scholar]
- 6. O’Reilly MS, Boehm T, Shing Y, et al. . Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 1997;88:277–85. 10.1016/S0092-8674(00)81848-6 [DOI] [PubMed] [Google Scholar]
- 7. Bloch W, Huggel K, Sasaki T, et al. . The angiogenesis inhibitor endostatin impairs blood vessel maturation during wound healing. Faseb J 2000;14:2373–6. 10.1096/fj.00-0490fje [DOI] [PubMed] [Google Scholar]
- 8. Wenzel D, Schmidt A, Reimann K, et al. . Endostatin, the proteolytic fragment of collagen XVIII, induces vasorelaxation. Circ Res 2006;98:1203–11. 10.1161/01.RES.0000219899.93384.ed [DOI] [PubMed] [Google Scholar]
- 9. Zügel M, Qiu S, Laszlo R, et al. . The role of sex, adiposity, and gonadectomy in the regulation of irisin secretion. Endocrine 2016;54:101–10. 10.1007/s12020-016-0913-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kjaer M, Langberg H, Heinemeier K, et al. . From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand J Med Sci Sports 2009;19:500–10. 10.1111/j.1600-0838.2009.00986.x [DOI] [PubMed] [Google Scholar]
- 11. Miller BF, Hansen M, Olesen JL, et al. . Tendon collagen synthesis at rest and after exercise in women. J Appl Physiol 2007;102:541–6. 10.1152/japplphysiol.00797.2006 [DOI] [PubMed] [Google Scholar]
- 12. Magnusson SP, Hansen M, Langberg H, et al. . The adaptability of tendon to loading differs in men and women. Int J Exp Pathol 2007;88:237–40. 10.1111/j.1365-2613.2007.00551.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fede C, Albertin G, Petrelli L, et al. . Hormone receptor expression in human fascial tissue. Eur J Histochem 2016;60:2710 10.4081/ejh.2016.2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hansen M, Kongsgaard M, Holm L, et al. . Effect of estrogen on tendon collagen synthesis, tendon structural characteristics, and biomechanical properties in postmenopausal women. J Appl Physiol 2009;106:1385–93. 10.1152/japplphysiol.90935.2008 [DOI] [PubMed] [Google Scholar]
- 15. Hansen M, Miller BF, Holm L, et al. . Effect of administration of oral contraceptives in vivo on collagen synthesis in tendon and muscle connective tissue in young women. J Appl Physiol 2009;106:1435–43. 10.1152/japplphysiol.90933.2008 [DOI] [PubMed] [Google Scholar]
- 16. Pavan PG, Stecco A, Stern R, et al. . Painful connections: densification versus fibrosis of fascia. Curr Pain Headache Rep 2014;18:441 10.1007/s11916-014-0441-4 [DOI] [PubMed] [Google Scholar]
- 17. Zhang C, Gao Y. Effects of aging on the lateral transmission of force in rat skeletal muscle. J Biomech 2014;47:944–8. 10.1016/j.jbiomech.2014.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kragstrup TW, Kjaer M, Mackey AL. Structural, biochemical, cellular, and functional changes in skeletal muscle extracellular matrix with aging. Scand J Med Sci Sports 2011;21:749–57. 10.1111/j.1600-0838.2011.01377.x [DOI] [PubMed] [Google Scholar]
- 19. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 2014;69(Suppl 1):S4–S9. 10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- 20. Calve S, Simon HG. Biochemical and mechanical environment cooperatively regulate skeletal muscle regeneration. Faseb J 2012;26:2538–45. 10.1096/fj.11-200162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pagel CN, Wasgewatte Wijesinghe DK, Taghavi Esfandouni N, et al. . Osteopontin, inflammation and myogenesis: influencing regeneration, fibrosis and size of skeletal muscle. J Cell Commun Signal 2014;8:95–103. 10.1007/s12079-013-0217-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shireman PK, Contreras-Shannon V, Ochoa O, et al. . MCP-1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. J Leukoc Biol 2007;81:775–85. 10.1189/jlb.0506356 [DOI] [PubMed] [Google Scholar]
- 23. Wang H, Melton DW, Porter L, et al. . Altered macrophage phenotype transition impairs skeletal muscle regeneration. Am J Pathol 2014;184:1167–84. 10.1016/j.ajpath.2013.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mackey AL, Kjaer M, Dandanell S, et al. . The influence of anti-inflammatory medication on exercise-induced myogenic precursor cell responses in humans. J Appl Physiol 2007;103:425–31. 10.1152/japplphysiol.00157.2007 [DOI] [PubMed] [Google Scholar]
- 25. Christensen B, Dandanell S, Kjaer M, et al. . Effect of anti-inflammatory medication on the running-induced rise in patella tendon collagen synthesis in humans. J Appl Physiol 2011;110:137–41. 10.1152/japplphysiol.00942.2010 [DOI] [PubMed] [Google Scholar]
- 26. Mackey AL, Rasmussen LK, Kadi F, et al. . Activation of satellite cells and the regeneration of human skeletal muscle are expedited by ingestion of nonsteroidal anti-inflammatory medication. Faseb J 2016;30:2266–81. 10.1096/fj.201500198R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trappe TA, Carroll CC, Dickinson JM, et al. . Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol 2011;300:R655–R662. 10.1152/ajpregu.00611.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tidball JG. Force transmission across muscle cell membranes. J Biomech 1991;24(Suppl 1):43–52. 10.1016/0021-9290(91)90376-X [DOI] [PubMed] [Google Scholar]
- 29. HUIJING PA. Intra-, extra- and intermuscular myofascial force transmision of synergists and antagonists: effects of muscle length as well as relative position. J Mech Med Biol 2002;02:405–19. 10.1142/S0219519402000496 [DOI] [Google Scholar]
- 30. Bojsen-Møller J, Schwartz S, Kalliokoski KK, et al. . Intermuscular force transmission between human plantarflexor muscles in vivo. J Appl Physiol 2010;109:1608–18. 10.1152/japplphysiol.01381.2009 [DOI] [PubMed] [Google Scholar]
- 31. Huijing PA, Yaman A, Ozturk C, et al. . Effects of knee joint angle on global and local strains within human triceps surae muscle: MRI analysis indicating in vivo myofascial force transmission between synergistic muscles. Surg Radiol Anat 2011;33:869–79. 10.1007/s00276-011-0863-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tian M, Herbert RD, Hoang P, et al. . Myofascial force transmission between the human soleus and gastrocnemius muscles during passive knee motion. J Appl Physiol 2012;113:517–23. 10.1152/japplphysiol.00111.2012 [DOI] [PubMed] [Google Scholar]
- 33. Yaman A, Ozturk C, Huijing PA, et al. . Magnetic resonance imaging assessment of mechanical interactions between human lower leg muscles in vivo. J Biomech Eng 2013;135:091003 10.1115/1.4024573 [DOI] [PubMed] [Google Scholar]
- 34. Herbert RD, Hoang PD, Gandevia SC. Are muscles mechanically independent? J Appl Physiol 2008;104:1549–50. 10.1152/japplphysiol.90511.2008 [DOI] [PubMed] [Google Scholar]
- 35. Maas H, Sandercock T. Force transmission between synergistic skeletal muscles through connective tissue linkages. J Biomed Biotechnol 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bernabei M, van Dieën JH, Maas H. Altered mechanical interaction between rat plantar flexors due to changes in intermuscular connectivity. Scand J Med Sci Sports 2017;27:177–87. 10.1111/sms.12644 [DOI] [PubMed] [Google Scholar]
- 37. Smeulders MJ, Kreulen M. Myofascial force transmission and tendon transfer for patients suffering from spastic paresis: a review and some new observations. J Electromyogr Kinesiol 2007;17:644–56. 10.1016/j.jelekin.2007.02.002 [DOI] [PubMed] [Google Scholar]
- 38. Yucesoy CA, Huijing PA. Substantial effects of epimuscular myofascial force transmission on muscular mechanics have major implications on spastic muscle and remedial surgery. J Electromyogr Kinesiol 2007;17:664–79. 10.1016/j.jelekin.2007.02.008 [DOI] [PubMed] [Google Scholar]
- 39. Huijing PA, Voermans NC, Baan GC, et al. . Muscle characteristics and altered myofascial force transmission in tenascin-X-deficient mice, a mouse model of Ehlers-Danlos syndrome. J Appl Physiol 2010;109:986–95. 10.1152/japplphysiol.00723.2009 [DOI] [PubMed] [Google Scholar]
- 40. Hinz B, Phan SH, Thannickal VJ, et al. . Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol 2012;180:1340–55. 10.1016/j.ajpath.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schleip R, Duerselen L, Vleeming A, et al. . Strain hardening of fascia: static stretching of dense fibrous connective tissues can induce a temporary stiffness increase accompanied by enhanced matrix hydration. J Bodyw Mov Ther 2012;16:94–100. 10.1016/j.jbmt.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 42. Kääriäinen M, Järvinen T, Järvinen M, et al. . Relation between myofibers and connective tissue during muscle injury repair. Scand J Med Sci Sports 2000;10:332–7. 10.1034/j.1600-0838.2000.010006332.x [DOI] [PubMed] [Google Scholar]
- 43. Smith LR, Lee KS, Ward SR, et al. . Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J Physiol 2011;589:2625–39. 10.1113/jphysiol.2010.203364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ramaswamy KS, Palmer ML, van der Meulen JH, et al. . Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats. J Physiol 2011;589:1195–208. 10.1113/jphysiol.2010.201921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wilke J, Krause F, Vogt L, et al. . What is evidence-based about myofascial chains: a systematic review. Arch Phys Med Rehabil 2016;97:454–61. 10.1016/j.apmr.2015.07.023 [DOI] [PubMed] [Google Scholar]
- 46. Vleeming A, Pool-Goudzwaard AL, Stoeckart R, et al. . The posterior layer of the thoracolumbar fascia. Its function in load transfer from spine to legs. Spine 1995;20:753–8. 10.1097/00007632-199504000-00001 [DOI] [PubMed] [Google Scholar]
- 47. Yucesoy CA, Baan GC, Koopman BH, et al. . Pre-strained epimuscular connections cause muscular myofascial force transmission to affect properties of synergistic EHL and EDL muscles of the rat. J Biomech Eng 2005;127:819–28. 10.1115/1.1992523 [DOI] [PubMed] [Google Scholar]
- 48. Stecco A, Antonio S, Gilliar W, et al. . The anatomical and functional relation between gluteus maximus and fascia lata. J Bodyw Mov Ther 2013;17:512–7. 10.1016/j.jbmt.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 49. Wilke J, Niederer D, Vogt L, et al. . Remote effects of lower limb stretching: preliminary evidence for myofascial connectivity? J Sports Sci 2016;34:2145–8. 10.1080/02640414.2016.1179776 [DOI] [PubMed] [Google Scholar]
- 50. Wilke J, Vogt L, Niederer D, et al. . Is remote stretching based on myofascial chains as effective as local exercise? A randomised-controlled trial. J Sports Sci 2017;35 10.1080/02640414.2016.1251606 [DOI] [PubMed] [Google Scholar]
- 51. Mooney V, Pozos R, Vleeming A, et al. . Exercise treatment for sacroiliac pain. Orthopedics 2001;24:29–32. [DOI] [PubMed] [Google Scholar]
- 52. Fedorczyk JM, Barr AE, Rani S, et al. . Exposure-dependent increases in IL-1beta, substance P, CTGF, and tendinosis in flexor digitorum tendons with upper extremity repetitive strain injury. J Orthop Res 2010;28:298–307. 10.1002/jor.20984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Barr AE, Barbe MF. Inflammation reduces physiological tissue tolerance in the development of work-related musculoskeletal disorders. J Electromyogr Kinesiol 2004;14:77–85. 10.1016/j.jelekin.2003.09.008 [DOI] [PubMed] [Google Scholar]
- 54. Gao HG, Fisher PW, Lambi AG, et al. . Increased serum and musculotendinous fibrogenic proteins following persistent low-grade inflammation in a rat model of long-term upper extremity overuse. PLoS One 2013;8:e71875 10.1371/journal.pone.0071875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Barbe MF, Gallagher S, Popoff SN. Serum biomarkers as predictors of stage of work-related musculoskeletal disorders. J Am Acad Orthop Surg 2013;21:644–6. 10.5435/JAAOS-21-10-644 [DOI] [PubMed] [Google Scholar]
- 56. Frara N, Fisher PW, Zhao Y, et al. . Substance P increases CCN2 dependent on TGF-beta yet Collagen Type I via TGF-beta1 dependent and independent pathways in tenocytes. Connective tissue research 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Driscoll M, Blyum L. The presence of physiological stress shielding in the degenerative cycle of musculoskeletal disorders. J Bodyw Mov Ther 2011;15:335–42. 10.1016/j.jbmt.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 58. Fisher PW, Zhao Y, Rico MC, et al. . Increased CCN2, substance P and tissue fibrosis are associated with sensorimotor declines in a rat model of repetitive overuse injury. J Cell Commun Signal 2015;9:37–54. 10.1007/s12079-015-0263-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xin DL, Hadrévi J, Elliott ME, et al. . Effectiveness of conservative interventions for sickness and pain behaviors induced by a high repetition high force upper extremity task. BMC Neurosci 2017;18:36 10.1186/s12868-017-0354-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang H, Schiltenwolf M, Buchner M. The role of TNF-alpha in patients with chronic low back pain-a prospective comparative longitudinal study. Clin J Pain 2008;24:273–8. 10.1097/AJP.0b013e31816111d3 [DOI] [PubMed] [Google Scholar]
- 61. Klyne DM, Barbe MF, van den Hoorn W, et al. . ISSLS PRIZE IN CLINICAL SCIENCE 2018: longitudinal analysis of inflammatory, psychological, and sleep-related factors following an acute low back pain episode-the good, the bad, and the ugly. Eur Spine J 2018;27:763–77. 10.1007/s00586-018-5490-7 [DOI] [PubMed] [Google Scholar]
- 62. Gerber C, Meyer DC, Schneeberger AG, et al. . Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am 2004;86-A:1973–82. 10.2106/00004623-200409000-00016 [DOI] [PubMed] [Google Scholar]
- 63. Hodges P, Holm AK, Hansson T, et al. . Rapid atrophy of the lumbar multifidus follows experimental disc or nerve root injury. Spine 2006;31:2926–33. 10.1097/01.brs.0000248453.51165.0b [DOI] [PubMed] [Google Scholar]
- 64. Hides JA, Stokes MJ, Saide M, et al. . Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine 1994;19:165–72. 10.1097/00007632-199401001-00009 [DOI] [PubMed] [Google Scholar]
- 65. Hodges PW, Galea MP, Holm S, et al. . Corticomotor excitability of back muscles is affected by intervertebral disc lesion in pigs. Eur J Neurosci 2009;29:1490–500. 10.1111/j.1460-9568.2009.06670.x [DOI] [PubMed] [Google Scholar]
- 66. Hodges PW, James G, Blomster L, et al. . Multifidus muscle changes after back injury are characterized by structural remodeling of muscle, adipose and connective tissue, but not muscle atrophy: molecular and morphological evidence. Spine 2015;40:1057–71. 10.1097/BRS.0000000000000972 [DOI] [PubMed] [Google Scholar]
- 67. James G, Sluka KA, Blomster L, et al. . Macrophage polarization contributes to local inflammation and structural change in the multifidus muscle after intervertebral disc injury. European Spine Journal 2018;380 10.1007/s00586-018-5652-7 [DOI] [PubMed] [Google Scholar]
- 68. Hodges PW, James G, Blomster L, et al. . Can proinflammatory cytokine gene expression explain multifidus muscle fiber changes after an intervertebral disc lesion? Spine 2014;39:1010–7. 10.1097/BRS.0000000000000318 [DOI] [PubMed] [Google Scholar]
- 69. Bas S, Finckh A, Puskas GJ, et al. . Adipokines correlate with pain in lower limb osteoarthritis: different associations in hip and knee. Int Orthop 2014;38:2577–83. 10.1007/s00264-014-2416-9 [DOI] [PubMed] [Google Scholar]
- 70. Abdelmagid SM, Barr AE, Rico M, et al. . Performance of repetitive tasks induces decreased grip strength and increased fibrogenic proteins in skeletal muscle: role of force and inflammation. PLoS One 2012;7:e38359 10.1371/journal.pone.0038359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Berrueta L, Muskaj I, Olenich S, et al. . Stretching impacts inflammation resolution in connective tissue. J Cell Physiol 2016;231:1621–7. 10.1002/jcp.25263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bove GM, Harris MY, Zhao H, et al. . Manual therapy as an effective treatment for fibrosis in a rat model of upper extremity overuse injury. J Neurol Sci 2016;361:168–80. 10.1016/j.jns.2015.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. O’leary S, Jull G, Van Wyk L, et al. . Morphological changes in the cervical muscles of women with chronic whiplash can be modified with exercise-A pilot study. Muscle Nerve 2015;52:772–9. 10.1002/mus.24612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hides JA, Richardson CA, Jull GA. Multifidus muscle recovery is not automatic after resolution of acute, first-episode low back pain. Spine 1996;21:2763–9. 10.1097/00007632-199612010-00011 [DOI] [PubMed] [Google Scholar]
- 75. James G, Millecamps M, Stone LS, et al. . Dysregulation of the Inflammatory Mediators in the Multifidus Muscle After Spontaneous Intervertebral Disc Degeneration SPARC-null Mice is Ameliorated by Physical Activity. Spine 2018:1 10.1097/BRS.0000000000002656 [DOI] [PubMed] [Google Scholar]
- 76. Langevin HM, Sherman KJ. Pathophysiological model for chronic low back pain integrating connective tissue and nervous system mechanisms. Med Hypotheses 2007;68:74–80. 10.1016/j.mehy.2006.06.033 [DOI] [PubMed] [Google Scholar]
- 77. Schleip R, Wilke J, Schreiner S, et al. . Needle biopsy-derived myofascial tissue samples are sufficient for quantification of myofibroblast density. Clin Anat 2018;31:368–72. 10.1002/ca.23040 [DOI] [PubMed] [Google Scholar]
- 78. Jaffrin MY, Morel H. Body fluid volumes measurements by impedance: a review of bioimpedance spectroscopy (BIS) and bioimpedance analysis (BIA) methods. Med Eng Phys 2008;30:1257–69. 10.1016/j.medengphy.2008.06.009 [DOI] [PubMed] [Google Scholar]
- 79. Seffinger MA, Najm WI, Mishra SI, et al. . Reliability of spinal palpation for diagnosis of back and neck pain: a systematic review of the literature. Spine 2004;29:E413–25. [DOI] [PubMed] [Google Scholar]
- 80. Stochkendahl MJ, Christensen HW, Hartvigsen J, et al. . Manual examination of the spine: a systematic critical literature review of reproducibility. J Manipulative Physiol Ther 2006;29:475–85. 10.1016/j.jmpt.2006.06.011 [DOI] [PubMed] [Google Scholar]
- 81. Wilke J, Vogt L, Pfarr T, et al. . 2018. Reliability and validity of a semi-electronic tissue compliance meter to assess muscle stiffness. J Back Musculoskel Rehabil. 10.3233/BMR-170871 [DOI] [PubMed] [Google Scholar]
- 82. Fischer AA. Tissue compliance meter for objective, quantitative documentation of soft tissue consistency and pathology. Arch Phys Med Rehabil 1987;68:122–5. [PubMed] [Google Scholar]
- 83. Wilke J, Banzer W. Non-invasive screening of fascial tissues – a narrative review. Phys Med Rehab Kurort 2014;24:117–24. [Google Scholar]
- 84. Finnoff JT, Hall MM, Adams E, et al. . American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med 2015;49:145–50. 10.1136/bjsports-2014-094219 [DOI] [PubMed] [Google Scholar]
- 85. Drakonaki EE, Allen GM, Wilson DJ. Ultrasound elastography for musculoskeletal applications. Br J Radiol 2012;85:1435–45. 10.1259/bjr/93042867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mc Auliffe S, Mc Creesh K, Purtill H, et al. . A systematic review of the reliability of diagnostic ultrasound imaging in measuring tendon size: Is the error clinically acceptable? Phys Ther Sport 2017;26:S146630207–3. 10.1016/j.ptsp.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 87. Langevin HM, Fox JR, Koptiuch C, et al. . Reduced thoracolumbar fascia shear strain in human chronic low back pain. BMC Musculoskelet Disord 2011;12:203 10.1186/1471-2474-12-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Maganaris CN, Paul JP. In vivo human tendon mechanical properties. J Physiol 1999;521:307–13. 10.1111/j.1469-7793.1999.00307.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. DeWall RJ, Slane LC, Lee KS, et al. . Spatial variations in Achilles tendon shear wave speed. J Biomech 2014;47:2685–92. 10.1016/j.jbiomech.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Slane LC, Thelen DG. Achilles tendon displacement patterns during passive stretch and eccentric loading are altered in middle-aged adults. Med Eng Phys 2015;37:712–6. 10.1016/j.medengphy.2015.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fukunaga T, Kawakami Y, Kubo K, et al. . Muscle and tendon interaction during human movements. Exerc Sport Sci Rev 2002;30:106–10. 10.1097/00003677-200207000-00003 [DOI] [PubMed] [Google Scholar]
- 92. Wulf M, Wearing SC, Hooper SL, et al. . Achilles tendon loading patterns during barefoot walking and slow running on a treadmill: An ultrasonic propagation study. Scand J Med Sci Sports 2015;25:868–75. 10.1111/sms.12455 [DOI] [PubMed] [Google Scholar]
- 93. Wearing SC, Hooper SL, Smeathers JE, et al. . Tendinopathy alters ultrasound transmission in the patellar tendon during squatting. Scand J Med Sci Sports 2016;26:1415–22. 10.1111/sms.12602 [DOI] [PubMed] [Google Scholar]
- 94. Arampatzis A, Karamanidis K, Mademli L, et al. . Plasticity of the human tendon to short- and long-term mechanical loading. Exerc Sport Sci Rev 2009;37:66–72. 10.1097/JES.0b013e31819c2e1d [DOI] [PubMed] [Google Scholar]
- 95. Wiesinger HP, Kösters A, Müller E, et al. . Effects of increased loading on in vivo tendon properties: a systematic review. Med Sci Sports Exerc 2015;47:1885–95. 10.1249/MSS.0000000000000603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Reeves ND, Maganaris CN, Narici MV. Effect of strength training on human patella tendon mechanical properties of older individuals. J Physiol 2003;548:971–81. 10.1113/jphysiol.2002.035576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Epro G, Mierau A, Doerner J, et al. . The Achilles tendon is mechanosensitive in older adults: adaptations following 14 weeks versus 1.5 years of cyclic strain exercise. J Exp Biol 2017;220:1008–18. 10.1242/jeb.146407 [DOI] [PubMed] [Google Scholar]
- 98. Couppé C, Kongsgaard M, Aagaard P, et al. . Habitual loading results in tendon hypertrophy and increased stiffness of the human patellar tendon. J Appl Physiol 2008;105:805–10. 10.1152/japplphysiol.90361.2008 [DOI] [PubMed] [Google Scholar]
- 99. Stenroth L, Cronin NJ, Peltonen J, et al. . Triceps surae muscle-tendon properties in older endurance- and sprint-trained athletes. J Appl Physiol 2016;120:63–9. 10.1152/japplphysiol.00511.2015 [DOI] [PubMed] [Google Scholar]
- 100. Maganaris CN, Paul JP. Tensile properties of the in vivo human gastrocnemius tendon. J Biomech 2002;35:1639–46. 10.1016/S0021-9290(02)00240-3 [DOI] [PubMed] [Google Scholar]
- 101. Maganaris CN, Chatzistergos P, Reeves ND, et al. . Quantification of internal stress-strain fields in human tendon: unraveling the mechanisms that underlie regional tendon adaptations and mal-adaptations to mechanical loading and the effectiveness of therapeutic eccentric exercise. Front Physiol 2017;8:91 10.3389/fphys.2017.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lewis T, Cook J. Fluoroquinolones and tendinopathy: a guide for athletes and sports clinicians and a systematic review of the literature. J Athl Train 2014;49:422–7. 10.4085/1062-6050-49.2.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wilke J, Vogt L, Banzer W. Immediate effects of self-myofascial release on latent trigger point sensitivity: a randomized-placebo-controlled trial. Biol Sport. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Speed C. A systematic review of shockwave therapies in soft tissue conditions: focusing on the evidence. Br J Sports Med 2014;48:1538–42. 10.1136/bjsports-2012-091961 [DOI] [PubMed] [Google Scholar]
- 105. Douglas J, Pearson S, Ross A, et al. . Chronic adaptations to eccentric training: a systematic review. Sports Med 2017;47:917–41. 10.1007/s40279-016-0628-4 [DOI] [PubMed] [Google Scholar]
- 106. Schroeder AN, Best TM. Is self myofascial release an effective preexercise and recovery strategy? A literature review. Curr Sports Med Rep 2015;14:747–58. 10.1249/JSR.0000000000000148 [DOI] [PubMed] [Google Scholar]
- 107. Schroeder AN, Best TM. Is self myofascial release an effective preexercise and recovery strategy? A literature review. Curr Sports Med Rep 2015;14:200–8. 10.1249/JSR.0000000000000148 [DOI] [PubMed] [Google Scholar]
- 108. Aboodarda SJ, Greene RM, Philpott DT, et al. . The effect of rolling massage on the excitability of the corticospinal pathway. Appl Physiol Nutr Metab 2018;43 10.1139/apnm-2017-0408 [DOI] [PubMed] [Google Scholar]
- 109. Hotfiel T, Swoboda B, Krinner S, et al. . Acute effects of lateral thigh foam rolling on arterial tissue perfusion determined by spectral doppler and power doppler ultrasound. J Strength Cond Res 2017;31:893–900. 10.1519/JSC.0000000000001641 [DOI] [PubMed] [Google Scholar]
- 110. Franke H, Franke JD, Fryer G. Osteopathic manipulative treatment for nonspecific low back pain: a systematic review and meta-analysis. BMC Musculoskelet Disord 2014;15:286 10.1186/1471-2474-15-286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jacobson E. Structural integration, an alternative method of manual therapy and sensorimotor education. J Altern Complement Med 2011;17:891–9. 10.1089/acm.2010.0258 [DOI] [PMC free article] [PubMed] [Google Scholar]