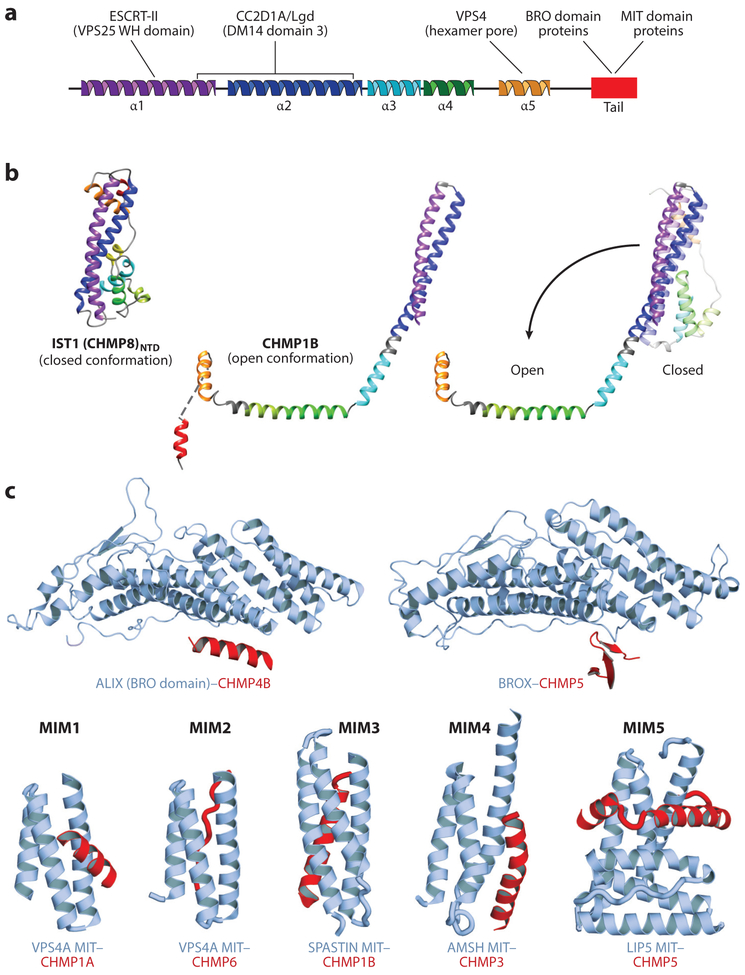
Figure 2. Structures and binding partners of the ESCRT-III proteins.
(a) Secondary structure showing the five conserved helices that organize ESCRT-III proteins in the closed conformation. The terminal ligand-binding tail (red) is helical in most ESCRT-III complexes but can alternatively adopt a β-strand secondary structure in some cases (see panel c). ESCRT-III ligands and their approximate binding sites are shown above the secondary structure, (b) Structures of ESCRT-III subunits in their open and closed configurations. (Left to right) Crystal structure of the ESCRT-III subunit IST1NTD in the closed conformation (from Bajorek et al. 2009), cryo-EM structure of CEIMP1B in the open conformation (from McCullough et al. 2015), and superposition of the closed (lighter shades, modeled) and open (darker shades) conformations of CHMP1B (from McCullough et al. 2015, Talledge et al. 2018). The N-terminal helical hairpin remains intact (and is extended upon opening), while the remaining helices either pack against the hairpin (closed conformation) or open to pack against other subunits in the CHMP1B filament (open conformation; see Figure 3b). (c) Structures of the C-terminal tails of ESCRT-III proteins (red) in complex with their two major classes of binding partners (blue-gray): BRO domain proteins such as ALIX and BROX (above) and MIT domain proteins such as VPS4, SPASTIN, AMSH, and LIP5 (Vta1) (below). Note the variety of distinct ways in which different ESCRT-III tails can bind BRO and MIT domains [denoted MIT-interacting motifs (MIMs) 1–5 in the MIT case]. Structures above are from McCullough et al. (2008) (left) and Mu et al. (2012) (right). Structures below (from left to right) are from Stuehell-Brereton et al. (2007), Kieffer et al. (2008), Yang et al. (2008), Solomons et al. (2011), and Skalieky et al. (2012).