Abstract
Objective
To compare the real-word effectiveness of subcutaneous tocilizumab (TCZ-SC) and intravenous tocilizumab (TCZ-IV) in rheumatoid arthritis (RA).
Methods
Patients with RA with TCZ from eight European registries were included. Drug retention was compared using unadjusted Kaplan-Meier and Cox models adjusted for baseline patient, disease and treatment characteristics, using a strata term for year of treatment initiation and country of registry. The proportions of patients achieving Clinical Disease Activity Index (CDAI) remission and low disease activity (LDA) at 1 year were compared using samples matched on the same covariates and corrected for attrition using LUNDEX.
Results
3448 patients were retrieved, 2414 with TCZ-IV and 1034 with TCZ-SC. Crude median retention was 3.52 years (95% CI 3.22 to 3.85) for TCZ-IV and 2.12 years for TCZ-SC (95% CI 1.88 to 2.38). In a country-stratified and year of treatment initiation–stratified, covariate-adjusted analysis, hazards of discontinuation were similar between TCZ-SC and TCZ-IV treated patients (HR 0.93, 95% CI 0.80 to 1.09). The average adjusted CDAI change at 1 year was similar in both groups (−6.08). After matching, with 560 patients in each group, CDAI remission corrected for attrition at 1 year was also similar between TCZ-SC and TCZ-IV (10.4% in TCZ-IV vs 12.8% in TCZ-SC (difference: 2.4%, bootstrap 95% CI −2.1% to 7.6%)), but CDAI LDA was lower in TCZ-IV patients: 41.0% in TCZ-IV versus 49.1% in TCZ-SC (difference: 8.0 %; bootstrap 95% CI 2.4% to 12.4%).
Conclusion
With similar retention and effectiveness, TCZ-SC is an adequate alternative to TCZ-IV for RA. When possible, considering the costs of the TCZ-IV route, TCZ-SC should be the preferred mode of administration.
Keywords: rheumatoid arthritis, biological therapies, DMARDs, tocilizumab, subcutaneous, intravenous, epidemiology
Key messages.
What is already known about this subject?
In randomised controlled trials of patients with rheumatoid arthritis, subcutaneous tocilizumab has been shown to be non-inferior to its intravenous formula.
What does this study add?
In this real-world setting register study with unselected patients with rheumatoid arthritis, subcutaneous tocilizumab and intravenous tocilizumab show comparable effectiveness. Switching from one mode of delivery to another did also not seem to alter effectiveness.
How might this impact on clinical practice?
When possible, considering the costs of the intravenous route, subcutaneous tocilizumab should be the preferred mode of administration.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune joint disease characterised by joint inflammation that may lead to structural damage. Since the development of biologic disease-modifying antirheumatic drugs (bDMARDs), its management and outcome have significantly changed with an important increase in the number of treatments available to achieve the desired target.1 Tocilizumab (TCZ) is a humanised anti-IL-6 receptor antibody that significantly decreases disease activity and inhibits structural damage, improving the patient’s outcome.2 3 Intravenous TCZ (TCZ-IV) has been licensed in Europe in 2009 for the management of patients with RA refractory to conventional synthetic (cs)DMARDs and tumour necrosis factor inhibitors, followed by the approval of subcutaneous (TCZ-SC) formulation in 2014.4 The efficacy and safety of TCZ-SC has been studied in different clinical trials. In BREVACTA, TCZ-SC was compared with placebo and had significantly greater efficacy on American College of Rheumatology (ACR) response rates and joint damage inhibition.5 In the MUSASHI clinical trial, 348 patients were randomised to receive either TCZ-SC or TCZ-IV in a double-blind design. The results showed that TCZ-SC was non-inferior to TCZ-IV in terms of efficacy and adverse events.6 In SUMMACTA, TCZ-SC was compared with TCZ-IV in 1262 patients with a 1:1 randomisation.7 At 97 weeks, the proportions of patients achieving ACR 20/50/70 responses and Disease Activity Score 28 (DAS28) remission were comparable in the two treatment arms.7 However, to our knowledge, the efficacy of these two modes of administration has not been compared in a real-world setting. Thus, the objective of this observational study is to evaluate the effectiveness of TCZ-IV and TCZ-SC in terms of retention and disease activity in patients with RA across eight European registries.
Methods
The TOCERRA collaboration of registries (TOcilizumab Collaboration of European Registries in RA) is an investigator-led project aiming at evaluating clinical aspects of TCZ use in patients with RA, supported by the industry, which has been described elsewhere.8 9 This study restricted the sample to the registries from the collaboration that contributed both TCZ-IV and TCZ-SC treated patients and thus comprised data from eight countries: Czech Republic (ATTRA), Finland (ROB-FIN), Italy (GISEA), Norway (NOR-DMARD), Portugal (Reuma.pt), Slovenia (BioRx.si), Spain (BIOBADASER) and Switzerland (SCQM). All patients starting treatment with TCZ between 1 January 2009 and 15 March 2018 were considered eligible for the present study. Inclusion criteria were diagnosis of RA established by a rheumatologist and baseline information on the mode of delivery.
Exposure of interest
The main exposure of interest was the mode of administration of TCZ (subcutaneous vs intravenous).
Study outcomes
Our main outcomes were drug retention and disease activity in terms of Clinical Disease Activity Index (CDAI) following initiation of TCZ. A secondary outcome was the disease activity in terms of DAS28.
Drug retention is supposed to reflect both effectiveness and tolerance of a drug and is reliably assessed in all registries.10 11 It was defined as the time from the start date of TCZ treatment until the treatment discontinuation date plus 1 month, as we estimated that the treatment was still effective during this time. If treatment had not been discontinued, we censored retention at the date of the last reported follow-up visit.
CDAI and DAS28 were analysed both as a continuous outcome over time and as a measure of remission (CDAI ≤2.8, DAS28 <2.6) or low disease activity (LDA, 2.8<CDAI≤10, 2.6≤DAS28<3.2) at 1 year.12–15 Considering the frequency of assessments in most of the registries, shorter evaluations of remission or LDA were not possible.
Covariates
The baseline covariates considered were sex, age, ever smoking, Body Mass Index (BMI) as a continuous variable and as a categorical variable (BMI<25, 25BMI<30, BMI30), disease duration, seropositivity (presence of rheumatoid factor (RF) or anticyclic citrullinated peptide antibodies (ACPA)), previously used bDMARDs, concomitant csDMARDs, use of glucocorticoids (GC) and daily dosage, functional disability (Health Assessment Questionnaire, HAQ), markers of disease activity (CDAI, DAS28), inflammation markers (erythrocyte sedimentation rate (ESR), C reactive protein (CRP)), presence of comorbidities (cardiovascular disease, interstitial lung disease, infection, malignancy and/or neuropsychiatric disorder), year of treatment initiation and country of registry. We did not adjust for level of education in the models as this covariate was missing for 57.8% of patients in the main analysis and completely missing in two registers. To limit misclassification of exposure and assign seronegative status to patients with missing data, seropositivity was defined as positive if RF and/or ACPA were positive according to each national registry, negative if both were negative and missing if one was missing and the other was negative.
Statistical methods
Baseline characteristics across delivery mode were compared using χ2 test for categorical variables and Wilcoxon rank-sum test for continuous variables. We analysed drug retention with Kaplan-Meier and Cox models. In the Cox models, the baseline hazards were allowed to vary by country of registers and year of treatment initiation. A post hoc analysis adjusting instead of stratifying for year of treatment initiation was added to explore its effect. A cluster term was added to account for patients with multiple courses of tocilizumab. Missing covariates were imputed using multiple imputations with chained equations, using 50 samples. CDAI and DAS28 change over time were analysed with mixed-effects models for longitudinal data, with a cubic effect of time, but Spain register was excluded for this analysis because of some key information missing. Because of collinearity between CDAI and DAS28, CRP and ESR, glucocorticoid use and dose, only CDAI, ESR and GC use were used for the models. The frequency of disease remission or LDA under treatment was assessed at 1 year post-treatment start. When no observed values within a 3-month window were available, they were imputed using a quadratic interpolation for each patient. We estimated the proportions of patients reaching remission or LDA by treatment group using proportions and compared them with the χ2 test. We also corrected for drug discontinuation using the LUNDEX index (index combining the proportion of patients fulfilling specific response criteria with the proportion of patients still adhering to therapy).16 The advantage of this index is that it integrates clinical response as well as adherence to therapy in a composite value. Thus, it avoids the selection only of patients who are still under treatment, excluding those who stopped for example for ineffectiveness or an adverse event and therefore resulting in a selection bias in favour of responders, which may overestimate drug effectiveness. To compare the LUNDEX between groups while adjusting for covariates, we also matched on a 1:1 basis the TCZ-SC and TCZ-IV population using a propensity score estimated on the same covariates as for the Cox model, except for CDAI, HAQ and ESR that were matched exactly after categorisation, with a calliper width of 0.2 of the pooled SD of the logit of the propensity score. CIs around the differences in LUNDEX-corrected remission or LDA rates were computed using bootstrap with 10 000 bootstrap samples. We did two sensitivity analyses: one taking into account only patients after the introduction in the market of TCZ-SC (2014) and another analysing separately patients depending on whether they switched or not their route of administration (which yielded four groups: TCZ-SC only, TCZ-IV only, TCZ-SC2IV for patients switching from TCZ-SC to TCZ-IV and TCZ-IV2SC for patients switching from TCZ-IV to TCZ-SC). All analyses and tabulations were performed using R V.3.4.2 with the mice, Survival, lme4, lmertest and Matching packages.
Results
A total of 3448 patients were retrieved before 15 March 2018, including 2414 with TCZ-IV and 1034 with TCZ-SC at baseline. All the registries contributed patients to both the TCZ-IV and TCZ-SC groups. Baseline demographics were similar between the groups, and there was no difference in the number of previous bDMARDs, but patients in the TCZ-IV group had more often methotrexate only than other csDMARDs or combination of csDMARDs and more severe baseline disease characteristics, including higher DAS28, HAQ, tender joint count (TJC), swollen joint count (SJC), ESR and CRP than patients treated with TCZ-SC (table 1). There were more patients with higher levels of education in the TCZ-SC population. For patients with TCZ-SC, the majority had 162 mg every 2 weeks. Only 2 patients had TCZ-SC every 10 days, 2 every 3 weeks and 31 every month. For patients with TCZ-IV, the majority of patients had a dose of 8 mg/kg with 54 patients who had a dose of 4 mg/kg. The standard interval was every 4 weeks with one patient who had TCZ-IV every 2 weeks and two every 3 weeks. Patient characteristics of the sensitivity analysis are available in online supplementary tables S1 and S2. In the TCZ-SC2IV group comprising only 18 patients, they could not be included in the discontinuation and disease activity analyses, and the TCZ-IV2SC population could also not be included in the bootstrap analysis, including only 274 patients.
Table 1.
Baseline characteristics
| N=3448 | TCZ-IV n=2414 | n | TCZ-SC n=1034 | n | P values |
| Total no of visits (median (IQR)) | 4 (2–8) | 2 (1–4) | |||
| Total patient-years | 5606.0 | 1273.5 | |||
| Age, years (median (IQR)) | 56.6 (47.7–64.5) | 2414 | 57.3 (48.6–65.3) | 1034 | 0.17 |
| Female gender, n (%) | 1923 (79.7) | 2414 | 850 (82.2) | 1034 | 0.09 |
| Ever smoker | 456 (32.1) | 1420 | 202 (29.0) | 696 | 0.16 |
| BMI (median (IQR)) | 25.6 (22.6–29.1) | 1950 | 26.2 (23.1–29.6) | 719 | 0.02 |
| Disease duration, years (median (IQR)) | 6.2 (2.7–12.3) | 1950 | 6.7 (2.8–13.0) | 747 | 0.39 |
| Seropositivity (RF and/or ACPA), n (%) | 1572 (80.0) | 2139 | 663 (81.4) | 814 | 0.41 |
| Previous bDMARDs, n (%) | 2266 | 964 | 0.47 | ||
| None | 692 (30.5) | 319 (33.1) | |||
| 1 | 921 (40.6) | 387 (40.1) | |||
| 2 | 474 (20.9) | 185 (19.2) | |||
| ≥3 | 179 (7.9) | 73 (7.6) | |||
| Glucocorticoids | 1436 (61.6) | 2332 | 605 (60.9) | 993 | 0.75 |
| Glucocorticoid dose, mg/day (median, IQR) | 5.0 (5.0–10.0) | 1075 | 5.0 (5.0–10.0) | 361 | 0.67 |
| Concomitant csDMARD | 2372 | 1020 | <0.001 | ||
| None | 675 (28.5) | 284 (27.8) | |||
| MTX | 960 (40.5) | 317 (31.1) | |||
| MTX+other csDMARDs | 295 (12.4) | 175 (17.2) | |||
| Other than MTX | 442 (18.6) | 244 (23.9) | |||
| DAS28 (median (IQR)) | 4.4 (3.4–5.9) | 2414 | 4.1 (3.3–5.5) | 1034 | <0.001 |
| CDAI (median (IQR)) | 17.0 (13.5–28.4) | 2414 | 17.0 (15.0–24.7) | 1034 | 0.1 |
| HAQ (median (IQR)) | 1.1 (0.9–1.7) | 2414 | 1.0 (1.0–1.5) | 1034 | 0.02 |
| TJC (over 28 joints) (median (IQR)) | 8.0 (3.0–14.0) | 1933 | 6.0 (2.0–11.0) | 887 | <0.001 |
| SJC (over 28 joints) (median (IQR)) | 6.0 (2.0–10.0) | 1936 | 4.0 (2.0–8.0) | 890 | <0.001 |
| PGA (median (IQR)) | 57.0 (10.0–80.0) | 1653 | 60.0 (25.0–78.0) | 646 | 0.24 |
| PhGA (median (IQR)) | 40.0 (6.0–68.0) | 1540 | 40.0 (13.5–60.0) | 639 | 0.98 |
| ESR (mm/hour) (median (IQR)) | 27.0 (10.0–46.0) | 1770 | 22.0 (10.0–41.0) | 658 | 0.02 |
| CRP (mg/L) (median (IQR)) | 9.0 (3.0–25.8) | 1921 | 6.7 (2.0–19.3) | 863 | <0.001 |
| Education category | 1101 | 361 | 0.03 | ||
| 0–10 years | 394 (35.8) | 127 (35.2) | |||
| 11–13 years | 538 (48.9) | 158 (43.8) | |||
| >13 years | 169 (15.3) | 76 (21.1) | |||
| Comorbidities | 1310 (61.5) | 2129 | 524 (58.4) | 897 | 0.12 |
| No of patients for each route of delivery since 2014 by year of treatment initiation | |||||
| 2014 | 348 (80.9 %) | 82 (19.1%) | |||
| 2015 | 260 (41.9%) | 361 (58.1%) | |||
| 2016 | 183 (35.3%) | 336 (64.7%) | |||
| 2017–2018 | 91 (28.7%) | 226 (71.3%) | |||
ACPA, anti–citrullinated protein antibody; BMI, body mass index; CDAI, Clinical Disease Activity Index; DAS28, Disease Activity Score 28; ESR, erythrocyte sedimentation rate; HAQ, Health Assessment Questionnaire; IQR, interquartile range; MTX, methotrexate; PGA, patient global assessment; PhGA, Physician global assessment; RF, rheumatoid factor; SJC, swollen joint counts; TCZ-IV, intravenous tocilizumab; TCZ-SC, subcutaneous tocilizumab; TJC, tender joint counts; bDMARDs, biological disease-modifyingantirheumatic drugs; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs.
rmdopen-2018-000809supp001.pdf (366.9KB, pdf)
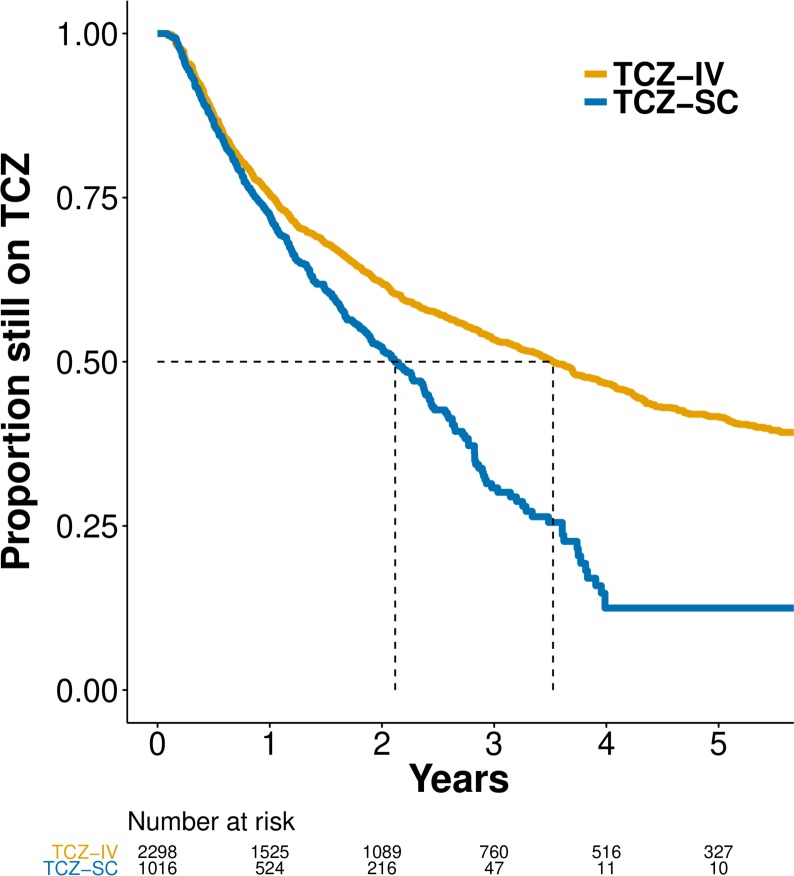
Crude median retention (figure 1) was 3.52 years (95% CI 3.22 to 3.85) for TCZ-IV and 2.12 years for TCZ-SC (95% CI 1.88 to 2.38). Crude retention was not significantly different between categories of BMI. In a country-stratified and year of treatment initiation–stratified, covariate-adjusted analysis, we found that hazards of discontinuation were similar among patients on TCZ-SC compared with patients on TCZ-IV (HR 0.93, 95% CI 0.80 to 1.09; table 2). Higher CDAI, higher HAQ and presence of GC at baseline were associated with greater risk of discontinuation; combination therapy with any csDMARDs, and in particular the combination of MTX or MTX and at least one other csDMARD, was associated with reduced risk of discontinuation, but not combination therapy of TCZ with other csDMARDs than MTX (table 2). The HR strongly swayed to 1 after stratification by year of treatment initiation, suggesting that this was the most important confounding factor linked to the difference in treatment discontinuation between TCZ-SC and TCZ-IV (online supplementary table S3). To explore this effect of year of treatment initiation, we added a post hoc analysis adjusting for calendar year (instead of stratifying) that showed increasing HR of discontinuation with more recent years (online supplementary table S3).
Figure 1.
Unadjusted Kaplan-Meier curves of drug discontinuation by route of administration. TCZ, tocilizumab; TCZ-IV, intravenous tocilizumab; TCZ-SC, subcutaneous tocilizumab.
Table 2.
Multivariable analysis of drug discontinuation
| HR | 95% CI | P values | |
| TCZ-IV | – | – | – |
| TCZ-SC | 0.93 | 0.80 to 1.09 | 0.37 |
| Age, years | 1.00 | 1.00 to 1.00 | 0.85 |
| Female gender | 0.96 | 0.84 to 1.09 | 0.54 |
| Disease duration, years | 1.00 | 1.00 to 1.01 | 0.32 |
| Seropositivity | 0.89 | 0.77 to 1.03 | 0.12 |
| BMI | 1.01 | 1.00 to 1.02 | 0.22 |
| Ever smoking | 1.11 | 0.97 to 1.28 | 0.14 |
| Glucocorticoids | 1.16 | 1.03 to 1.31 | 0.02 |
| Concomitant csDMARD (base=none) | 0.88 | 0.78 to 0.98 | 0.03 |
| Any csDMARD MTX | 0.87 | 0.76 to 1.00 | 0.04 |
| MTX+other csDMARDs | 0.79 | 0.66 to 0.94 | 0.008 |
| Other than MTX | 0.97 | 0.84 to 1.12 | 0.67 |
| Previous bDMARDs (base=none) | |||
| 1 | 1.06 | 0.94 to 1.20 | 0.37 |
| 2 | 1.10 | 0.94 to 1.29 | 0.25 |
| ≥3 | 1.10 | 0.88 to 1.38 | 0.39 |
| CDAI at baseline | 1.01 | 1.00 to 1.02 | 0.001 |
| HAQ at baseline | 1.14 | 1.04 to 1.26 | 0.008 |
| ESR | 1.00 | 1.00 to 1.00 | 0.53 |
| Comorbidities | 0.99 | 0.87 to 1.13 | 0.87 |
bDMARD, biological disease-modifying antirheumatic drug; BMI, Body Mass Index; csDMARD, conventional synthetic disease-modifying antirheumatic drug; CDAI, Clinical Disease Activity Index; ESR, erythrocyte sedimentation rate; HAQ, Health Assessment Questionnaire; MTX, methotrexate; TCZ-IV, intravenous tocilizumab; TCZ-SC, subcutaneous tocilizumab.
When looking at the year of prescription, there was a progressive increase in the use of TCZ-SC compared with TCZ-IV with more patients initiating TCZ-SC than TCZ-IV since 2015 (table 1, last rows). In the first sensitivity analysis taking only patients starting TCZ since 2014, the crude median retention was still higher in TCZ-IV, although the difference was less important (2.79 years, 95% CI 2.28 to 3.16 vs 2.01 years, 95% CI 1.80 to 2.27 for TCZ-SC) (online supplementary figure S1). After adjustment, the hazards of discontinuation were similar (HR 1.00, 95% CI 0.85 to 1.17). In this subset, absence of GC at baseline, co-therapy with MTX or MTX and at least another csDMARD at baseline were also associated with reduced risk of discontinuation. In the second sensitivity analysis, the crude median retention was still significantly higher between TCZ-IV only (3.12 years, 95% CI 2.76 to 3.47) and TCZ-SC only (2.12 years, 95% CI 1.89 to 2.38), and was even higher for TCZ-IV2SC (7.94 years, 95% CI 6.96 to NA, the upper endpoint of the 95% CI never reaching 50% of discontinuation) (online supplementary figure S2). After adjustment, the hazards of discontinuation when comparing TCZ-SC only to TCZ-IV only were not significantly different (HR 0.95, 95% CI 0.81 to 1.11 for TCZ-SC only). However, when comparing TCZ-IV-only patients to TCZ-IV2SC patients, hazards of discontinuation were still lower in the switching group (HR 0.65, 95% CI 0.56 to 0.76).
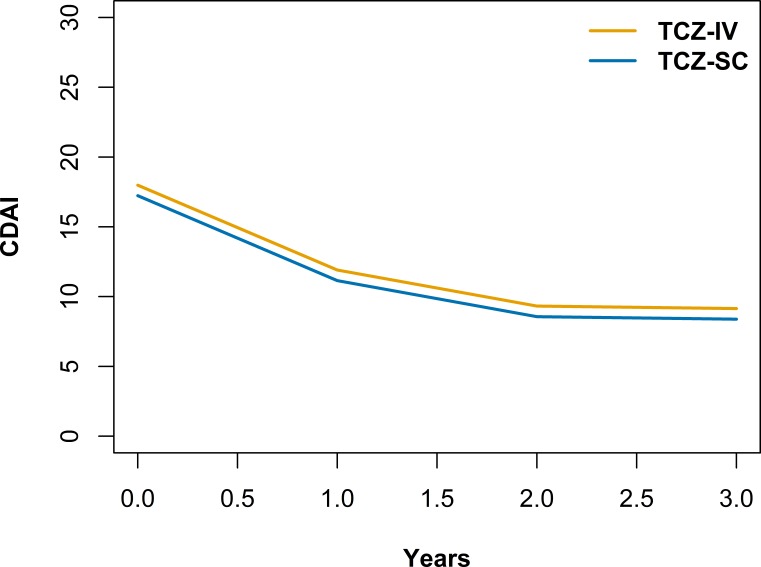
CDAI score and DAS28 decreased over time, faster initially before plateauing at around 2 years, for both modes of TCZ administration (figure 2, table 3 and online supplementary table S4). The average adjusted CDAI (p=0.12) and its evolution (p=0.38) was not significantly different between groups and the difference of CDAI between baseline and 1 year was −6.08. The average adjusted DAS28 was slightly lower in the TCZ-SC group (−0.15, 95% CI −0.26 to −0.05, p=0.004), but its evolution was not statistically different (p=0.16). Presence of GC, presence of a concomitant csDMARD, higher HAQ and higher ESR at baseline were associated with higher CDAI and DAS28 at any time during follow-up. Seropositivity was associated with a lower CDAI and DAS28 at any time during follow-up. Female gender was associated with higher DAS28 at any time during follow-up. The results were similar in the sensitivity analysis taking only patients starting TCZ since 2014 except that in the 2014 and after subset, the presence of concomitant csDMARD at baseline was no longer significantly associated with higher CDAI and DAS28 at any time during follow-up and that BMI was statistically associated with a slightly higher CDAI and DAS28 at any time during follow-up (data not shown). The average adjusted CDAI and its evolution also did not significantly differ between TCZ-IV only, TCZ-SC only, TCZ-IV only and TCZ-IV2SC.
Figure 2.
Multivariable analysis of Clinical Disease Activity Index (CDAI) over time modelled with a cubic effect of time and adjusted for age, gender, disease duration, seropositivity, Body Mass Index, smoking, presence of glucocorticoids, presence and type of concomitant conventional synthetic disease-modifying antirheumatic drugs (DMARDs), number of previous biologic DMARDs, Health Assessment Questionnaire score, erythrocyte sedimentation rate and presence of a comorbidity at baseline. TCZ-IV, intravenous tocilizumab; TCZ-SC, subcutaneous tocilizumab.
Table 3.
Multivariable analysis of CDAI over time
| Overall | |||
| Coeff | 95% CI | P values | |
| Treatment at baseline | |||
| TCZ-IV | – | – | |
| TCZ-SC | −0.76 | −1.70 to 0.19 | 0.12 |
| Time, years | −8.19 | −8.87 to −7.51 | <0.001 |
| Time-squared | 2.29 | 2.05 to 2.54 | <0.001 |
| Time-cubed | −0.18 | −0.21 to −0.16 | <0.001 |
| Age, years | −0.02 | −0.06 to 0.01 | 0.22 |
| Female gender | −0.54 | −1.66 to 0.58 | 0.34 |
| Disease duration, years | −0.01 | −0.07 to 0.05 | 0.72 |
| Seropositivity | −1.85 | −3.01 to −0.70 | 0.002 |
| BMI | 0.03 | −0.05 to 0.12 | 0.45 |
| Smoking | −0.32 | −1.20 to 0.55 | 0.47 |
| Glucocorticoids | 1.68 | 1.01 to 2.34 | <0.001 |
| Concomitant csDMARD (base=none) | 1.04 | 0.41 to 1.68 | 0.001 |
| Any csDMARD MTX | 1.51 | 0.81 to 2.21 | <0.001 |
| MTX+other csDMARDs | 0.70 | −0.60 to 2.00 | 0.29 |
| Other than MTX | −0.07 | −1.04 to 0.90 | 0.88 |
| Previous bDMARDs (base=none) | |||
| 1 | 0.42 | −0.63 to 1.48 | 0.43 |
| 2 | 0.80 | −0.36 to 1.96 | 0.18 |
| ≥3 | 0.06 | −1.63 to 1.74 | 0.95 |
| HAQ at baseline | 3.87 | 3.25 to 4.50 | <0.001 |
| ESR | 0.25 | 0.24 to 0.27 | <0.001 |
| Comorbidity | 0.17 | −0.80 to 1.14 | 0.73 |
bDMARD, biological disease-modifying antirheumatic drug; BMI, Body Mass Index; csDMARD, conventional synthetic disease-modifying antirheumatic drug; CDAI, Clinical Disease Activity Index; ESR, erythrocyte sedimentation rate; HAQ, Health Assessment Questionnaire; MTX, methotrexate; TCZ-IV, intravenous tocilizumab; TCZ-SC, subcutaneous tocilizumab.
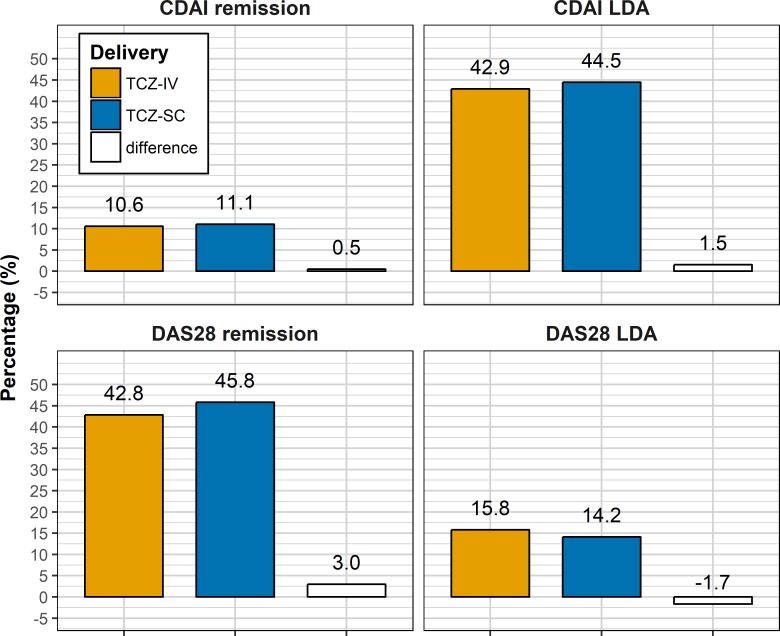
At 1 year, 1525 TCZ-IV and 524 TCZ-SC patients were still under treatment. Among them, 13.9% of TCZ-IV and 15.3% of TCZ-SC patients were in CDAI remission, 56.8% and 61.5% in CDAI not in remission but LDA, 56.6% and 63.4% in DAS28 remission, and 20.9% and 19.6% in DAS28 not in remission but LDA, respectively. There were no statistically significant differences in these responses rates between TCZ-SC and TCZ-IV except for the DAS28 remission, which was slightly higher in the TCZ-SC group (p=0.03), even after correction for attrition with the LUNDEX (figure 3, CDAI remission: 10.6% in TCZ-IV vs 11.1% in TCZ-SC (difference: 0.5%, bootstrap 95% CI −1.9% to 4.0%); CDAI LDA: 42.9% in TCZ-IV vs 44.5% in TCZ-SC (difference: 1.5%, bootstrap 95% CI −1.1% to 8.2%); DAS28 remission: 42.8% in TCZ-IV vs 45.8% in TCZ-SC (difference: 3.0%, bootstrap 95% CI 0.4 to 9.6); DAS28 LDA: 15.8% in TCZ-IV vs 14.2% in TCZ-SC (difference: −1.7%, bootstrap 95% CI −4.2 to 2.5)). After matching, there was no longer a significant difference of ESR and HAQ between the groups with 560 patients in each group (online supplementary table S5). From those, LUNDEX-corrected CDAI remission at 1 year in the matched population were not significantly different between TCZ-SC and TCZ-IV patients (CDAI remission: 10.4% in TCZ-IV vs 12.8% in TCZ-SC (difference: 2.4%, bootstrap 95% CI −2.1% to 7.6 %)), but CDAI LDA rates were higher in the TCZ-SC patients: 41.0% in TCZ-IV versus 49.1% in TCZ-SC (difference: 8.0%; bootstrap 95% CI 2.4% to 17.6%). The results were similar when including only patients since 2014, but CDAI LDA rates were not significantly different between TCZ-IV-only and TCZ-SC-only patients. CDAI remission and LDA rates were also similar between BMI categories, and there was no significant difference in the LUNDEX-corrected CDAI remission and LDA when comparing TCZ-IV and TCZ-SC by categories of BMI.
Figure 3.
LUNDEX-corrected Clinical Disease Activity Index (CDAI) and Disease Activity Score 28 (DAS28) remission and low disease activity (LDA). TCZ-IV, intravenous tocilizumab; TCZ-SC, subcutaneous tocilizumab.
For TCZIV2SC patients, the median time to switch was 2.21 years, 95% CI 0.92 to 3.47, and the median time of follow-up after switch was 1.16 years, 95% CI 0.08 to 2.56. For TCZSC2IV patients, the median time to switch was 0.5 years, 95% CI 0.06 to 1.14, and the median time of follow-up after switch was 0.36 years, 95% CI 0.08 to 1.13. CDAI decreased from baseline until the patients switched to the other route of administration and continued to decrease thereafter in a manner similar to the patients who did not switch (online supplementary table S6).
In patients where the reason for stopping the drug was collected, 35% in TCZ-SC stopped for adverse events, 36% for ineffectiveness and 28% for other reasons (which may include a combination of reasons, pregnancy or remission). In the TCZ-IV group, more patients stopped for other reason (34%) and ineffectiveness (36%) than for adverse events (26%). However, the cause of treatment discontinuation was not provided in more than 50% of patients in each group (56% in TCZ-IV and 75% in TCZ-SC).
Discussion
Our study is the first to compare TCZ-SC versus TCZ-IV in a real-world RA population. The results show that even if the crude drug discontinuation rate was very different, after adjustment, drug retention and clinical effectiveness, as assessed by CDAI and DAS28 changes and responses, were similar between TCZ-SC and TCZ-IV. These data indicate that TCZ-SC is an adequate alternative to TCZ-IV and confirm previous results from clinical trials in a large observational setting.6 7 To note, CDAI LDA was slightly higher in the TCZ-SC group.
Concerning the patient population, baseline demographic characteristics were very similar, but TCZ-IV patients had higher disease activity in terms of DAS28, HAQ, TJC, SJC, ESR and CRP. This may be linked to a channelling bias with TCZ-IV prescribed for more severe patients or to the fact that patients received TCZ-IV at an earlier period, when less alternatives were available and less accent was put on the treat-to-target strategy,17 leading to overall higher disease activity. Despite these baseline characteristics’ differences, the effectiveness was similar between TCZ-IV and TCZ-SC.
The difference in the crude retention between TCZ-SC and TCZ-IV was mainly associated to the treatment year of initiation. This important confounding effect of the calendar year has been described several times before in registries data and may be linked to the increased possibility of treatment or different expectations from the patients and physicians.18–20
As described before in TCZ and other bDMARDs, we found that a higher HAQ and disease activity at baseline were associated with higher discontinuation.19–21 Co-therapy with a csDMARD, especially MTX, was associated with a higher CDAI and DAS28 at any point during follow-up. This may be linked to a residual confounding effect of severity, patients with co-therapy having higher parameters of disease activity and severity at baseline (higher DAS28, PGA, PhGA, TJC and number of previous bDMARDs).
In our cohort, seropositivity was associated to a lower CDAI at any time during follow-up. A higher remission rate has been described in RF-positive patients treated with TCZ, but this was not reproduced in ACPA-positive patients in another cohort.22 23
Retention was higher with TCZ-IV2SC than TCZ-IV-only patients. Unfortunately, our data did not allow us to evaluate the reasons of switching between routes of delivery. It is possible that this reflects a selected population with better effectiveness, tolerance and compliance, who switched for practical reasons but were already considered as responders. These results are in line with two previous studies looking at patients switching from TCZ-IV to TCZ-SC. In the 84-week, open-label extension of the MUSASHI trial, 160 patients switched from TCZ-IV to TCZ-SC and efficacy was maintained after switch.24 Another small trial with 57 Japanese patients did not find a change of efficacy after switching from TCZ-IV to TCZ-SC after a follow-up of 3 months.25 However, our study is the first to evaluate switching from TCZ-IV to TCZ-SC in a large non-Japanese population. In the TCZ-SC2IV subgroup, the descriptive analysis of CDAI also showed a similar trend in efficacy before and after switch than other subgroups; however, the TCZ-SC2IV subgroup was too small and the duration of follow-up too short to draw any definitive conclusion.
As in previous studies, we did not find an effect of BMI on both TCZ formulations as assessed by drug retention or clinical effectiveness.25–27
Our study has several limitations. Data quality of registries is often less thorough than in clinical trials. In particular, we do not have enough details on causes of discontinuation or switching to allow further analysis. We have also no data on adherence that may be different between the TCZ-IV and TCZ-SC. Adherence is reported to be lower with subcutaneous bDMARDs and more frequent dosing schedule.28 29 Despite this, we did not find a difference in effectiveness when comparing TCZ-SC with TCZ-IV. Finally, we do not have data outside of the bDMARDs courses and thus could not explore other aspects as for example the rate of biologic-free remission between groups.
The strengths of this study is that we have a large population and number of covariates, a longer duration of follow-up and an observational setting which allow less strict inclusion criteria than in previous clinical trials who compared TCZ-SC with TCZ-IV.
In conclusion, our study shows that TCZ-SC is an adequate alternative to TCZ-IV in RA. Thus, when possible and considering the costs of administration of TCZ-IV, TCZ-SC should be the preferred mode of administration.
Acknowledgments
The authors thank all the rheumatologists and patients who participated in the registries.
Footnotes
Contributors: All the authors have provided substantial contributions to the conception or design of the work, the acquisition of the data and the interpretation of data. KL and DSC performed the statistical analysis. KL, DSC, DM and CG made the first draft. All the other authors participated in the final drafting of the work or revising it critically for important intellectual content. All authors contributed to the final approval of the version published.
Funding: The TOCERRA collaboration is funded by Roche. Clinical work in Czech Republic was partially supported by the project from the Ministry of Health for conceptual development of research organization MZ00023728 (Institute of Rheumatology). ROB-FIN is funded by AbbVie, Hospira, BMS, MSD, Pfizer, Roche and UCB. NOR-DMARD was previously supported with research funding to Diakonhjemmet Hospital from AbbVie, BMS, MSD/Schering-Plough, Pfizer/Wyeth, Roche and UCB. Reuma.pt is supported by unrestricted grants from Abbvie, Biogen, Celgene, MSD, Roche, Sanofi and Pfizer. BioRx.si has received funding for clinical research paid to Društvo za razvoj revmatologije from AbbVie, Roche, Medis, MSD and Pfizer. BIOBADASER has received funding from Fundacion Española de Reumatología, the Spanish Medicines and Health Products Agency (Agencia Española del Medicamento y Productos Sanitarios) and equal grants from pharmaceutical companies (AbbVie, Pfizer, Roche, Schering‐Plough and BMS). Swiss Clinical Quality Management in Rheumatic Diseases (SCQM) database is sponsored by public and industrial support (http://scqm.ch/en/sponsoren/).
Competing interests: KL: none declared. DM: none declared. FI: none declared. EKK: none declared. TKK has received fees for speaking and/or consulting from AbbVie, BMS, Celgene, Celltrion, Eli Lilly, Hospira, Merck-Serono, MSD, Orion Pharma, Pfizer, Roche, Sandoz and UCB, and received research funding to Diakonhjemmet Hospital from AbbVie, BMS, MSD, Pfizer, Roche and UCB. DN benefited from grant and research support from AbbVie, BMS, MSD, Pfizer, Roche and UCB, and has received fees for speaking and/or consulting for AbbVie, BMS, MSD, Roche, UCB and Pfizer. KP benefited from grant and research support from AbbVie, Roche, Medis, MSD and Pfizer, and has received fees for speaking and/or consulting for AbbVie, Roche, Amgen, MSD, BMS, UCB and Egis. MP-S: none declared. ZR: none declared. MJS: none declared. CC: has received speaker and consulting fees from AbbVie, Amgen, Angellini, Astra Zeneca, BMS, Egis, MSD, Pfizer, Richter, Roche, Sanofi, Servier, Teva, UCB and Zentiva. GL has received fees for consulting for BMS, Roche, MSD, AbbVie and Pfizer. DSC has received consulting fees from BMS, Pfizer and Janssen. CG has received fees for speaking and/or consulting from AbbVie, BMS, Roche, Pfizer, Celgene, MSD, Janssen Cilag, Amgen and UCB, and received research funding from Roche, AbbVie, MSD and Pfizer.
Patient consent: Obtained.
Ethics approval: Geneva Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All the data belong to the registries. Anonymised data can be shared as long as agreements are made with all participating registries.
References
- 1.Smolen JS, Landewé R, Bijlsma J, et al. . EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. 10.1136/annrheumdis-2016-210715 [DOI] [PubMed] [Google Scholar]
- 2.Maini RN, Taylor PC, Szechinski J, et al. . Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum 2006;54:2817–29. 10.1002/art.22033 [DOI] [PubMed] [Google Scholar]
- 3.Kremer JM, Blanco R, Brzosko M, et al. . Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate. Arthritis Rheum 2011;63:609–21. [DOI] [PubMed] [Google Scholar]
- 4.European Medicines Agency RoActemra.
- 5.Kivitz A, Olech E, Borofsky M, et al. . Subcutaneous tocilizumab versus placebo in combination with disease-modifying antirheumatic drugs in patients with rheumatoid arthritis. Arthritis Care Res 2014;66:1653–61. 10.1002/acr.22384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogata A, Tanimura K, Sugimoto T, et al. . Phase III study of the efficacy and safety of subcutaneous versus intravenous tocilizumab monotherapy in patients with rheumatoid arthritis. Arthritis Care Res 2014;66:344–54. 10.1002/acr.22110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burmester GR, Rubbert-Roth A, Cantagrel A, et al. . Efficacy and safety of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional DMARDs in patients with RA at week 97 (SUMMACTA). Ann Rheum Dis 2016;75:68–74. 10.1136/annrheumdis-2015-207281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lauper K, Nordström DC, Pavelka K, et al. . Comparative effectiveness of tocilizumab versus TNF inhibitors as monotherapy or in combination with conventional synthetic disease modifying antirheumatic drugs in patients with rheumatoid arthritis after the use of at least one biological disease modify. Ann Rheum Dis 2018:1–7. [DOI] [PubMed] [Google Scholar]
- 9.Gabay C, Riek M, Hetland ML, et al. . Effectiveness of tocilizumab with and without synthetic disease-modifying antirheumatic drugs in rheumatoid arthritis: results from a European collaborative study. Ann Rheum Dis 2016;75:1336–42. 10.1136/annrheumdis-2015-207760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pincus T, Marcum SB, Callahan LF. Longterm drug therapy for rheumatoid arthritis in seven rheumatology private practices: II. Second line drugs and prednisone. J Rheumatol 1992;19:1885–94. [PubMed] [Google Scholar]
- 11.Fisher A, Bassett K, Wright JM, et al. . Comparative persistence of the TNF antagonists in rheumatoid arthritis—a population-based cohort study. PLoS One 2014;9:e105193 10.1371/journal.pone.0105193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prevoo ML, van 't Hof MA, Kuper HH, et al. . Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. 10.1002/art.1780380107 [DOI] [PubMed] [Google Scholar]
- 13.Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol 2005;23(5 Suppl 39):S100–8. [PubMed] [Google Scholar]
- 14.Aletaha D, Nell VP, Stamm T, et al. . Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther 2005;7:R796–806. 10.1186/ar1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aletaha D, Ward MM, Machold KP, et al. . Remission and active disease in rheumatoid arthritis: defining criteria for disease activity states. Arthritis Rheum 2005;52:2625–36. 10.1002/art.21235 [DOI] [PubMed] [Google Scholar]
- 16.Kristensen LE, Saxne T, Geborek P. The LUNDEX, a new index of drug efficacy in clinical practice: results of a five-year observational study of treatment with infliximab and etanercept among rheumatoid arthritis patients in southern Sweden. Arthritis Rheum 2006;54:600–6. 10.1002/art.21570 [DOI] [PubMed] [Google Scholar]
- 17.Grigor C, Capell H, Stirling A, et al. . Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 2004;364:263–9. 10.1016/S0140-6736(04)16676-2 [DOI] [PubMed] [Google Scholar]
- 18.Du Pan SM, Dehler S, Ciurea A, et al. . Comparison of drug retention rates and causes of drug discontinuation between anti-tumor necrosis factor agents in rheumatoid arthritis. Arthritis Rheum 2009;61:560–8. 10.1002/art.24463 [DOI] [PubMed] [Google Scholar]
- 19.Neovius M, Arkema EV, Olsson H, et al. . Drug survival on TNF inhibitors in patients with rheumatoid arthritis comparison of adalimumab, etanercept and infliximab. Ann Rheum Dis 2015;74:354–60. 10.1136/annrheumdis-2013-204128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabay C, Riek M, Scherer A, et al. . Effectiveness of biologic DMARDs in monotherapy versus in combination with synthetic DMARDs in rheumatoid arthritis: data from the Swiss Clinical Quality Management Registry. Rheumatology 2015;54:1664–72. 10.1093/rheumatology/kev019 [DOI] [PubMed] [Google Scholar]
- 21.Forsblad-d'Elia H, Bengtsson K, Kristensen LE, et al. . Drug adherence, response and predictors thereof for tocilizumab in patients with rheumatoid arthritis: results from the Swedish biologics register. Rheumatology 2015;54:1186–93. 10.1093/rheumatology/keu455 [DOI] [PubMed] [Google Scholar]
- 22.Kawashiri SY, Kawakami A, Iwamoto N, et al. . In rheumatoid arthritis patients treated with tocilizumab, the rate of clinical disease activity index (CDAI) remission at 24 weeks is superior in those with higher titers of IgM-rheumatoid factor at baseline. Mod Rheumatol 2011;21:370–4. 10.3109/s10165-010-0409-0 [DOI] [PubMed] [Google Scholar]
- 23.Cappelli LC, Palmer JL, Kremer J, et al. . Tocilizumab treatment leads to improvement in disease activity regardless of CCP status in rheumatoid arthritis. Semin Arthritis Rheum 2017;47:165–9. 10.1016/j.semarthrit.2017.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogata A, Atsumi T, Fukuda T, et al. . Sustainable efficacy of switching from intravenous to subcutaneous tocilizumab monotherapy in patients with rheumatoid arthritis. Arthritis Care Res 2015;67:1354–62. 10.1002/acr.22598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwamoto N, Fukui S, Umeda M, et al. . Evaluation of switching from intravenous to subcutaneous formulation of tocilizumab in patients with rheumatoid arthritis. Mod Rheumatol 2016;26:662–6. 10.3109/14397595.2015.1129692 [DOI] [PubMed] [Google Scholar]
- 26.Pers YM, Godfrin-Valnet M, Lambert J, et al. . Response to tocilizumab in rheumatoid arthritis is not influenced by the body mass index of the patient. J Rheumatol 2015;42:580–4. 10.3899/jrheum.140673 [DOI] [PubMed] [Google Scholar]
- 27.Gardette A, Ottaviani S, Sellam J, et al. . Body mass index and response to tocilizumab in rheumatoid arthritis: a real life study. Clin Rheumatol 2016;35:857–61. 10.1007/s10067-016-3183-3 [DOI] [PubMed] [Google Scholar]
- 28.Calvo-Alén J, Monteagudo I, Salvador G, et al. . Non-adherence to subcutaneous biological medication in patients with rheumatoid arthritis: a multicentre, non-interventional study. Clin Exp Rheumatol 2017;35:423–30. [PubMed] [Google Scholar]
- 29.Mena-Vazquez N, Manrique-Arija S, Yunquera-Romero L, et al. . Adherence of rheumatoid arthritis patients to biologic disease-modifying antirheumatic drugs: a cross-sectional study. Rheumatol Int 2017;37:1709–18. 10.1007/s00296-017-3758-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2018-000809supp001.pdf (366.9KB, pdf)