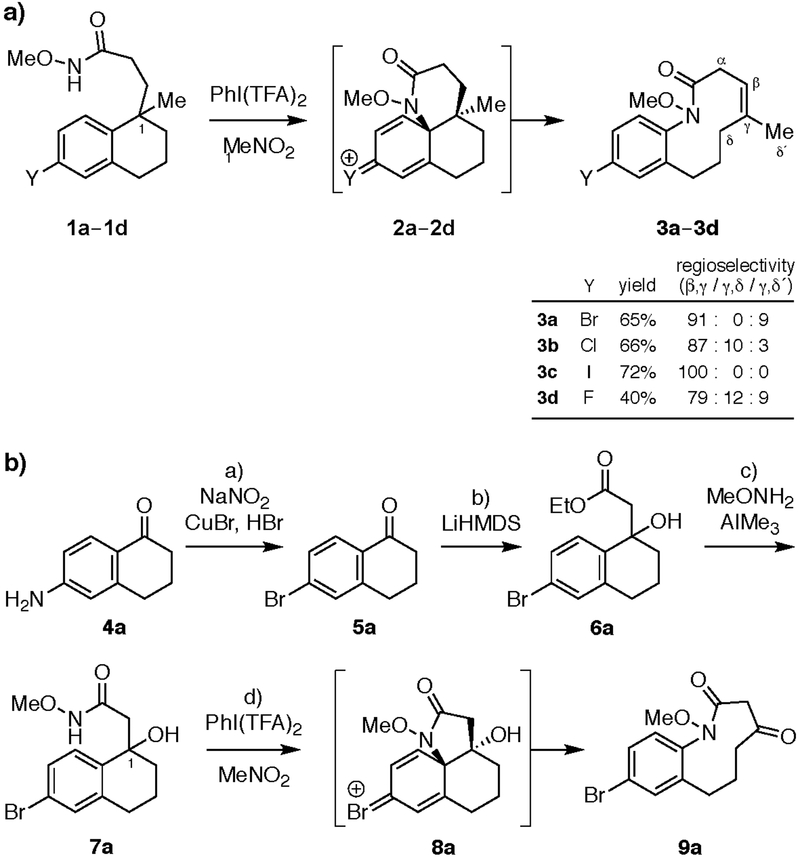
Figure 2. Tandem ODRE reactions of haloaromatic substrates to form medium-ring lactam products.
(a) Synthesis of 10-membered ring haloaromatics 3a–d (major isomer shown). Reagents and conditions: PhI(TFA)2 (2.0 equiv), MeNO2, 0 °C to 24 °C, 14 h. (b) Synthesis of 9- membered ketolactam 9a. Reagents and conditions: a) NaNO2 (1.1 equiv),CuBr (2.2 equiv), HBr (aq), 85%. b) LiHMDS (3.0 equiv), EtOAc (3.0 equiv), THF, –78 °C, 3 h, 93%. c) AlMe3 (3.0 equiv), NH2(OMe)・HCl (3.0 equiv), THF, 0 °C to 24 °C, 16 h, 96%. d) PhI(TFA)2 (1.5 equiv), MeNO2, 0 °C to 24 °C, 1 h, 73%. HMDS = hexamethyldisilazide; TFA = trifluoroacetate; THF = tetrahydrofuran.