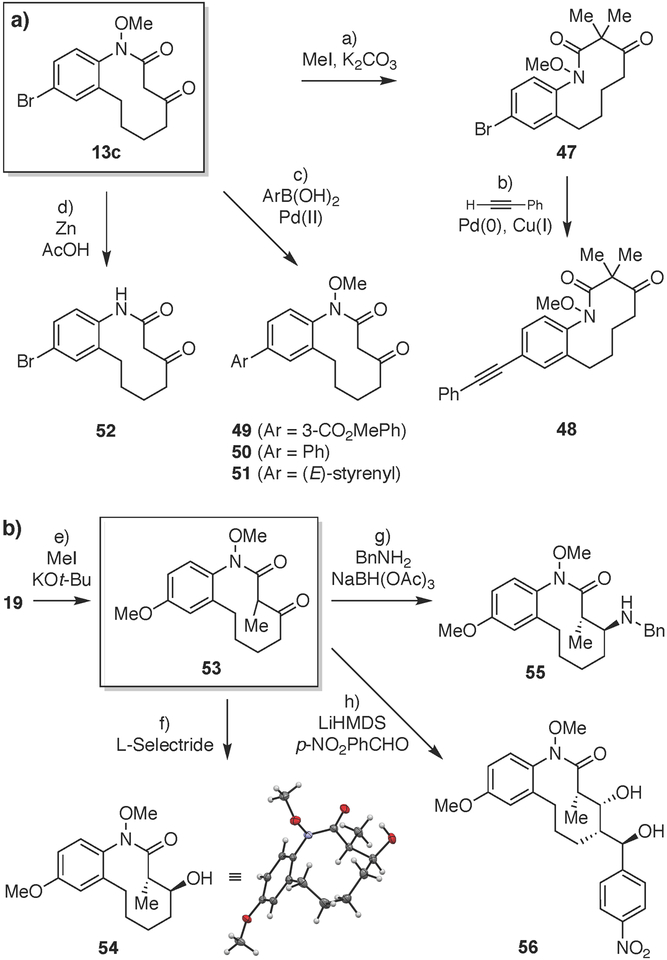
Figure 5. Downstream modification reactions of medium-ring scaffolds 13c and 53.
Reagents and conditions: a) Mel (4.0 equiv), K2CO3 (4.0 equiv), DMF, 24 °C, 48 h, 68%. b) phenylacetylene (10.0 equiv), Pd(PPh3)4 (20 mol%), Cul (25 mol%), Et3 N, DMF, 60 °C, 16 h, 65%. c) ArB(OH)2 (1.1 equiv), Pd(OAc)2 (20 mol%), K2CO3 (2.5 equiv), TBAB (1.1 equiv), H2O, 70 °C, 2 h, 65–77%. d) Zn (40.0 equiv), AcOH/H2O (1:1), 24 °C, 24 h, 85%. e) Mel (3.0 equiv), KOt-Bu (1.05 equiv), THF, 0 °C, 4 h, 72%. f) L-Selectride (2.0 equiv), THF, 0 °C to 24 °C, 3 h. 94%, >99:1 dr anti/syn. g) BnNH2 (1.1 equiv), AcOH (1.0 equiv), 4A MS, toluene, 90 °C, 2 h, then NaBH(OAc)3 (4.0 equiv), DCE, 24 °C, 16 h, 76%, 94:6 dr ant//syn. h) LiHMDS (3.0 equiv), THF, −78 °C, 1 h; then p-NO2 PhCHO (2.5 equiv), −78 °C to 24 °C, 16 h, 59%, 99:1 dr. DCE = 1,2-dichloroethane; DMF = N,N-dim ethylform amide; L-Selectride = lithium tri-s-butylborohydride; TBAB = tetrabutylamm onium bromide.