Figure 1.
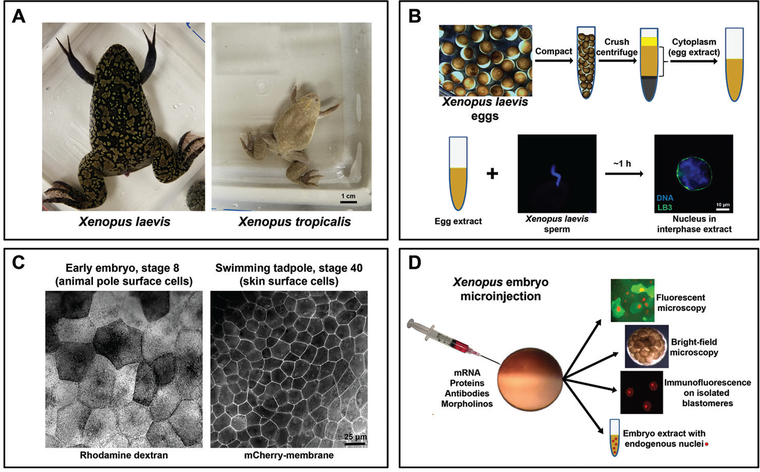
Xenopus is an important model system to study mechanisms and functions of nuclear size regulation. A. Comparison of adult frogs Xenopus laevis and Xenopus tropicalis. B. Eggs collected from the frog are compacted and fractionated by centrifugation, and cell-free cytoplasm is isolated. Addition of demembranated X. laevis sperm chromatin to egg extract triggers nuclear formation. LB3, lamin B3. C. Comparison of surface cells from a stage 8 embryo and stage 40 tadpole. The left image shows cells with fluorescent rhodamine-labeled dextran that serves as a tracer that was microinjected at the one-cell stage. The right image shows cells expressing mCherry fused to a membrane-targeting sequence expressed from mRNA microinjected at the one-cell stage. D. One-cell stage embryos can be microinjected with various materials to alter nuclear size, offering an excellent experimental approach to study the mechanisms and functional significance of nuclear size control. Live microinjected embryos expressing fluorescent proteins can be imaged by fluorescence microscopy to study changes in nuclear dynamics. Bright-field time lapse microscopy can be performed on whole microinjected embryos to quantify cell division timing. Single blastomeres can be isolated from embryos at different developmental stages for nuclear staining, imaging, and quantification. Different stage microinjected embryos can be used to generate embryo extracts containing endogenous nuclei, which can be further used for biochemical experiments, nuclear isolation, immunofluorescence studies, immunoblot analyses, etc.