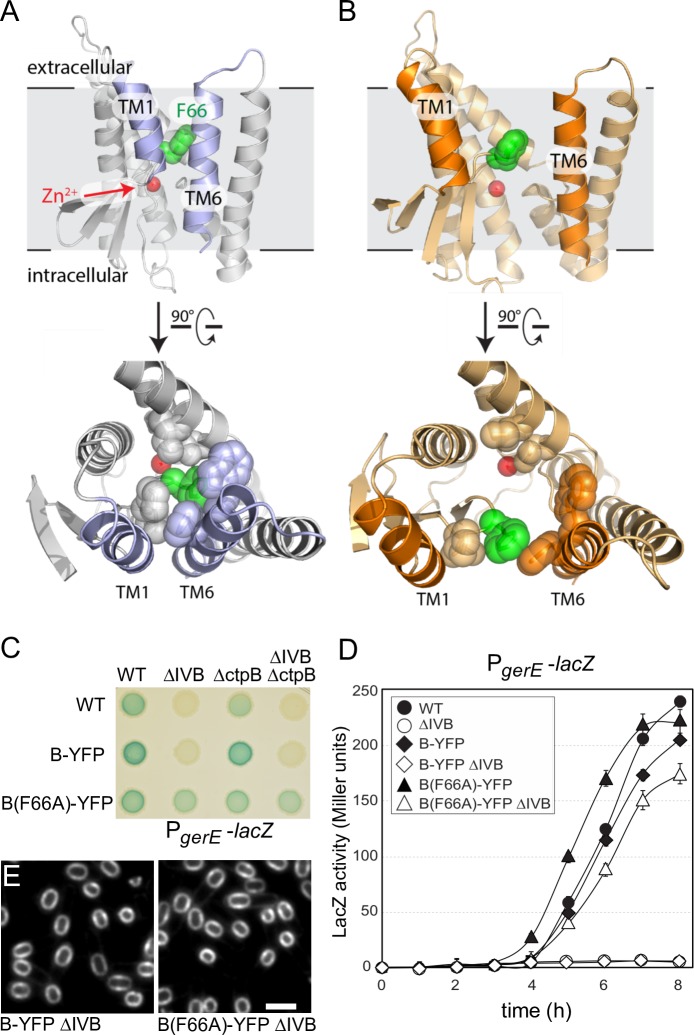
Fig 6. Evidence that B adopts open and closed conformations in vivo.
Models of the closed (A) and open (B) conformations of the B protease domain based on the mjS2P structures. Phenylalanine 66 (green) in the membrane-reentrant β-loop that is predicted to occlude substrate and help stabilize the closed conformation and the catalytic Zn2+ ion (red ball) are indicated. Open and closed conformations rotated 90˚ are shown below. The residues in the catalytic core that are within 5 Å of F66 in the closed conformation are shown in both states. (C) B(F66A)-YFP activates σK in the absence of IVB and an auxiliary signaling protease CtpB. σK activity was monitored using a σK-responsive promoter (PgerE) fused to lacZ. Image of a sporulation agar plate containing X-gal with the indicated strains after 24 hours of incubation at 37˚C. (D) Liquid β-galactosidase assay during a sporulation time course (n = 3). Only B(F66A) activates σK in the absence of IVB (open triangles). Liquid β-galactosidase assays of additional strains and immunoblot analysis of pro-σK processing can be found in S11 Fig. (E) B(F66A)-YFP localizes to the membranes surrounding the forespore. Representative images of the indicated strains at hour 4.5 of sporulation. Scale bar indicates 2 μm. Additional controls can be found in S12 Fig.