Figure 6.
The E3 Activity of UHRF1 Is Dependent on a Hydrophobic Patch on the UBL and a Regulatory Ubiquitin Binding Surface on the E2
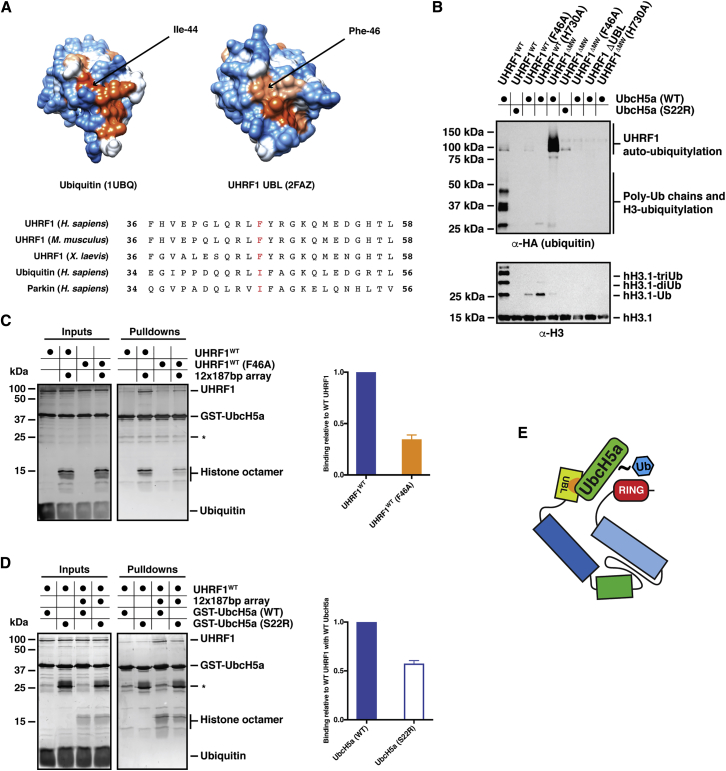
(A) (Top panel) Atomic space-fill structures of ubiquitin (PDB: 1UBQ) and the UHRF1 UBL domain (PDB: 2FAZ) color coded by hydrophobicity. Orange-red represents more hydrophobic residues, whereas blue residues are more polar. (Bottom panel) Sequence alignment of human ubiquitin with the UBL domain of UHRF1 from various species and the UBL domain of human Parkin. F46 in the UHRF1 UBL and corresponding isoleucines in ubiquitin and Parkin are indicated in red.
(B) E3 ubiquitin ligase assays carried out with the indicated mutants over 1 hr at 25°C. H3 ubiquitylation is drastically reduced in the F46A mutant with some UHRF1 auto-ubiquitylation observed. The S22R mutation in UbcH5a abrogates ubiquitylation of the substrate (UHRF1 or histone H3) in the context of both UHRF1WT and UHRF1ΔMW.
(C) GST-UbcH5a pull-downs using UHRF1WT and the F46A mutant. Binding assays were carried out as described in Figure 4C. Quantification of three independent experiments indicates reduced binding of F46A UHRF1 to UbcH5a relative to the wild-type in the presence of 12 × 187 bp arrays. The mean was plotted ± the SEM.
(D) GST-UbcH5a pull-downs using UHRF1WT with wild-type UbcH5a and the S22R mutant in the presence of 12 × 187 bp arrays. Quantification of three independent experiments reveals reduced binding of UHRF1 to S22R UbcH5a compared with the wild-type. The mean was plotted ± the SEM.
(E) Proposed model for the contact between the hydrophobic patch on the UBL domain of UHRF1 (indicated in orange) and the regulatory region on UbcH5a centered on Ser-22.