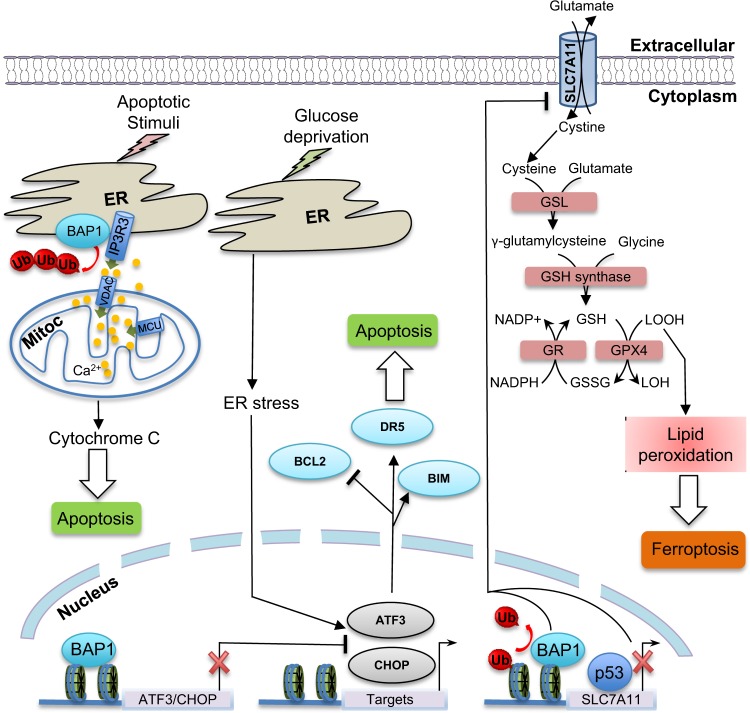
Fig. 1. Roles of the deubiquitylase and tumor suppressor BAP1 in cell death.
The tumor suppressor BAP1 promotes ferroptosis by repressing the expression of SLC7A11, a cystine/glutamate antiporter responsible for the cellular entry of cysteine, a metabolite used for the novo synthesis of reduced glutathione. Inhibition of SLC7A11 expression leads to low levels of reduced glutathione, diminished antioxidant capacity of the cells, thus resulting in Lipid-ROS accumulation and ferroptosis. BAP1 deubiquitylates and stabilizes the IP3R3 channel-receptor, thus promoting the release of Ca2+ from the endoplasmic reticulum (ER) into the cytoplasm and mitochondria (Mitoc). High levels of mitochondrial Ca2+, promote cytochrome c release from the mitochondria into the cytoplasm, a process that leads to apoptosis. Therefore, low levels of BAP1 inhibit both apoptosis and ferroptosis, facilitating the survival of DNA damage cells. BAP1 can also inhibit apoptosis by repressing the expression of key ER stress transcription factors with pro-apoptotic functions. The ability of BAP1 to modulate apoptosis and ferroptosis contributes to its tumor suppressor function in vivo. GSL: glutamate-cysteine ligase; GSH: Reduced glutathione; GSSG: oxidized glutathione; GR: Glutathione Reductase; GPX4: Glutathione Peroxidase 4; VDAC: Voltage-dependent anion channel; MCU: mitochondrial Ca2+ uniporter; Ub: Ubiquitin