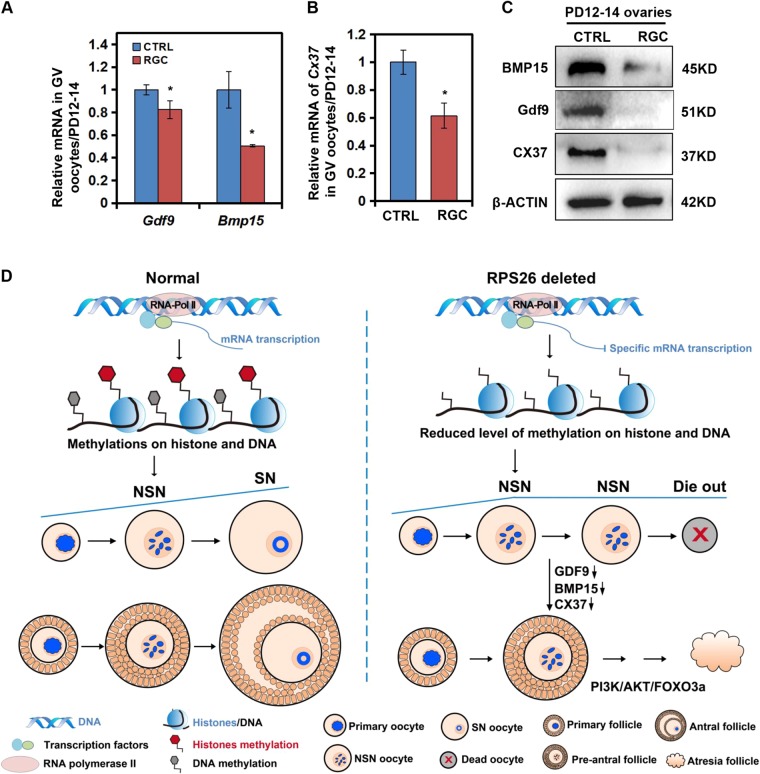
Fig. 7. Downregulation of the oocyte-derived factors Bmp15, Gdf9, and Cx37 and the proposed working model.
a The mRNA expression of Gdf9 and Bmp15 was decreased in oocytes of Rps26fl/fl/Gdf9-Cre (RGC) mice compared to control (CTRL) Rps26fl/fl mice. *p < 0.05 as calculated by two-tailed Student’s t-tests. b The mRNA expression of Cx37 was decreased in oocytes of Rps26fl/fl/Gdf9-Cre (RGC) mice compared to control (CTRL) Rps26fl/fl mice. *p < 0.05 as calculated by two-tailed Student’s t-tests. c Western blot results showing reduced expression of the Bmp15, Gdf9, and Cx37 proteins in PD12–14 ovaries from Rps26fl/fl/Gdf9-Cre (RGC) mice compared with control (CTRL) Rps26fl/fl mice. d The proposed working model. Generally, normal oocytes in primary follicles increase in mRNA synthesis activity mainly through RNA polymerase II, and they experience gradually increased levels of histone and DNA methylation, their nucleolus gradually transforms from the NSN to SN-type, and the follicles develop from pre-antral to antral follicles. Deletion of Rps26 in oocytes leads to reduced mRNA synthesis activity through downregulation of RNA polymerase II, and this leads to reduced levels of methylation modifications of histones and DNA and subsequent failure to transition from the NSN to SN-type. The oocytes secrete significantly lower levels of Gdf9, Bmp15, and Cx37, and this prevents follicle development from pre-antral follicles to antral follicles. In addition, inhibition of the PI3K/Akt/Foxo3a pathway in the ovary arrests the growth of NSN-type oocytes, and the oocytes finally die along with follicle degeneration, which ultimately leads to POF