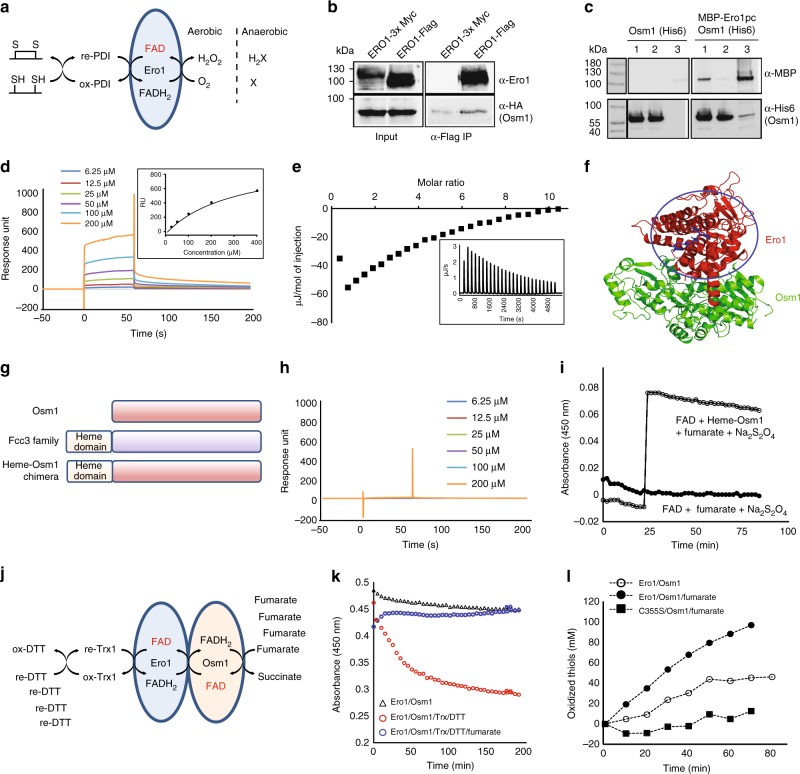
Fig. 4.
Physical interaction between Osm1 and Ero1. a Ero1 function. b Co-immunoprecipitation study. Cell lysates from yeast cells co-expressing Ero1-3×Myc or Ero1-FLAG were immunoprecipitated using anti-FLAG antibody. Samples were separated by SDS-PAGE and immunoblotted using anti-Ero1 antibody or anti-Osm1 serum. c In vitro pull-down assay. The interaction between Osm1 and Ero1 was analyzed by sequential pulldown with nickel then amylose beads. Samples were separated by SDS-PAGE and immunoblotted with anti-MBP and anti-6His antibodies. Lane 1: input; lane 2: fraction not bound to amylose beads; lane 3: 20× fraction bound to amylose beads. d Surface plasmon resonance. Purified MBP-Ero1 was immobilized and Osm1 was then injected over the surface. All sensorgrams were processed by subtracting the value of a blank sensorgram. Equilibrated response unit plotted against Osm1 concentration is shown in the black box. e The interaction between Osm1 and Ero1 confirmed by ITC. Integrated heat area of Ero1 was plotted by Osm1-to-Ero1 molar ratio. f Molecular docking of Osm1 with Ero1. g Domain composition of the Fcc3-Osm1 chimera. h Surface plasmon resonance. The purified Fcc3-Osm1 chimera was immobilized and Osm1 was then injected. i Reductase activity assay for the Fcc3-Osm1 chimera. Oxidation of FAD by 0.5 µM Fcc3-Osm1 chimera measured based on absorbance at 450 nm. j Model system used to assess Osm1-Ero1-mediated thiol oxidation. k, l In an airtight environment, when oxygen was depleted by overreaction between Ero1 and a reduced thiol mix (thioredoxin/DTT), the Ero1- and Osm1-bound FAD was reduced. However, fumarate inhibited this FAD reduction by working as an electron acceptor (k). At the same time, oxidized thiol molecules in reaction increased (l). The catalytically inactive Ero1 mutant, C355S, could not oxidize thiols even in the presence of Osm1 and fumarate