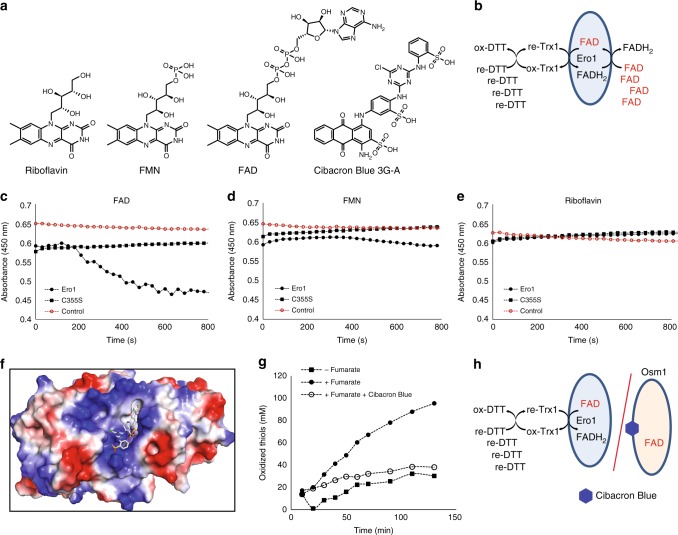
Fig. 5.
Effect of blocking the second FAD-binding pocket. a Chemical structures of riboflavin, FMN, FAD, and Cibacron Blue 3G-A. b Model system used to assess the ability of Ero1 to transfer electrons to free FAD. c–e Ero1 can only transfer electrons to FAD. Under airtight conditions, Ero1, or Ero1-C355S, was activated by adding a reduced thiol mix (DTT and thioredoxin) along with FAD (c), FMN (d), and riboflavin (e). Reduction of the flavin molecules was then monitored by changes in absorbance at 450 nm. f Docking simulation for Cibcron Blue 3G-A binding to Osm1. g Cibacron Blue 3G-A inhibits anaerobic thiol oxidation by the Ero1-Osm1 system. h Model system used to assess inhibition of disulfide bond formation by the Ero1-Osm1 system