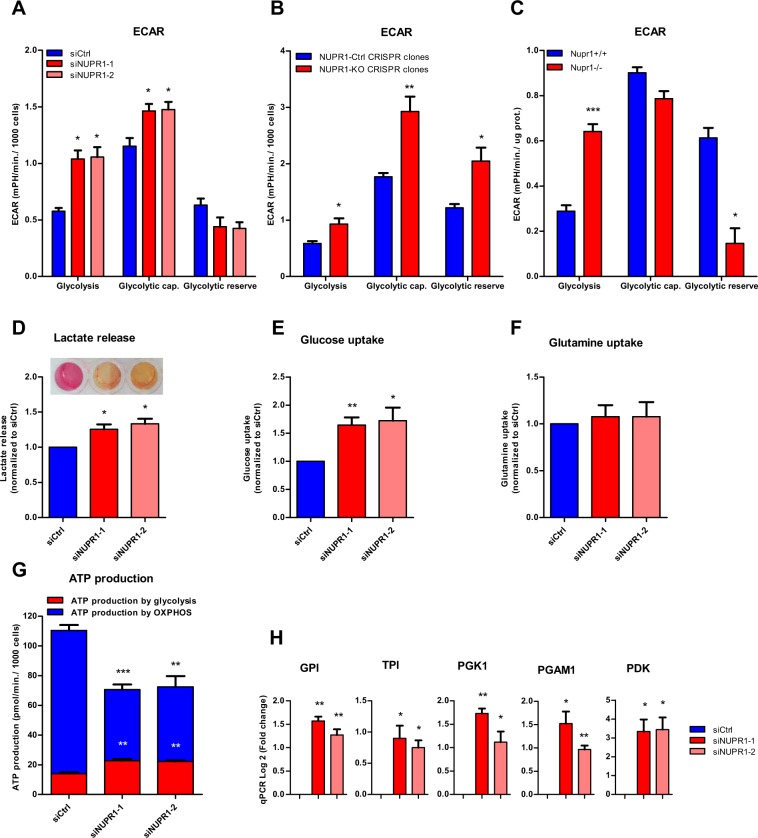
Figure 3.
Nupr1 deficiency promotes anaerobic glycolytic metabolism. Extracellular acidification rate (ECAR) levels were measured in MiaPaCa2 cells transfected with siCtrl, siNUPR1-1, or siNUPR1-2 for 72 h (A). The ECAR was measured in three clones of Panc-1 control cells (with wild-type Nupr1) and six clones of Nupr1 knockout cells, developed by CRISPR-Cas9 technology. Data are means of triplicates ± SEM of three control and six knockout clones (B). Statistically significant differences from Panc-1 control cells with p values ≤ 0.05 are shown. Also, the ECAR was measured in pancreatic acinar cells from WT (Nupr1+/+) and Nupr1−/− mice (C). The ECAR was measured under basal conditions or following the addition of glucose, oligomycin, or 2-Deoxyglucose. The rates of ECAR for glycolysis, glycolytic capacity, and glycolytic reserve were quantified as described in the Materials and Methods section. Lactate release (D), glucose (E) and glutamine (F) uptake and were measured in the extracellular medium after 24 h in culture. A representative image of DMEM collected after 72 h of culture is shown (D). ATP production by OXPHOS and anaerobic glycolysis were determined by the OCR and proton production rate, respectively (G); data values were normalized to the siCtrl. Total RNA was extracted to monitor mRNA levels of the genes involved in glycolysis using RT-qPCR (H). Data are means of triplicates ± SEM. For each treatment, statistically significant (*p < 0.05, **p < 0.01, ***p < 0.001) differences from siCtrl (A, D–H), Ctrl CRISPR clones (B) or Nupr1+/+ mice (C) are shown.