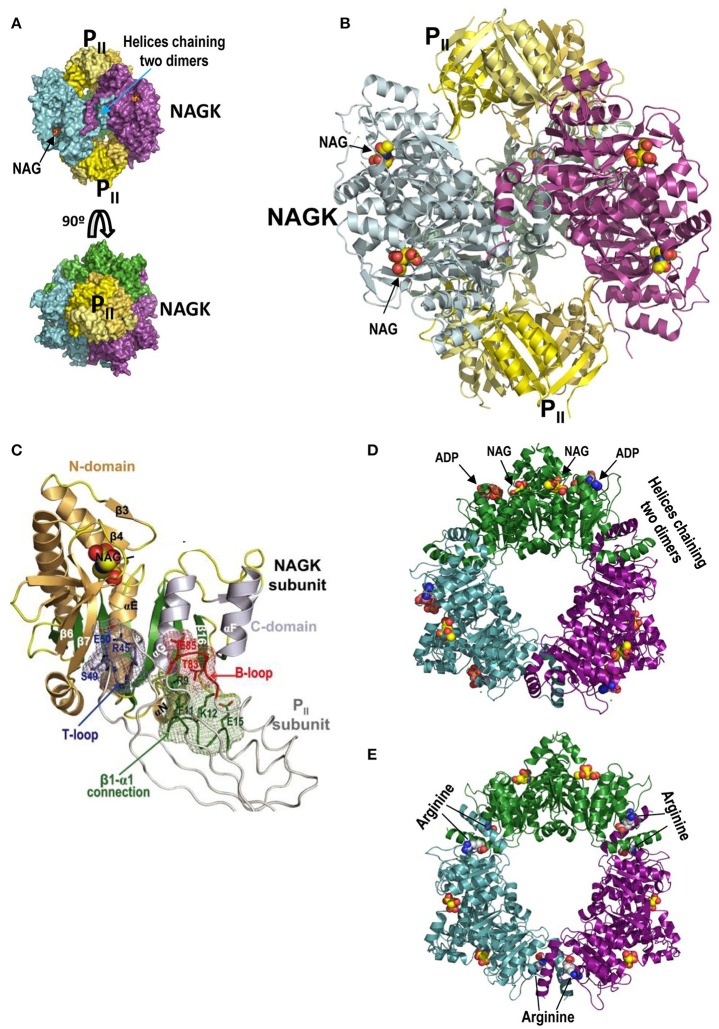
Figure 4.
PII-NAGK complex and active and arginine-inhibited NAGK. (A) The PII-NAGK complex of S. elongatus (PDB 2V5H; Llácer et al., 2010). Surface representations of the complex formed by two PII trimers (yellow) capping on both ends the doughnut-like NAGK hexamer (trimer of dimers; each dimer in a different color). The three-fold axis is vertical (top) or perpendicular to the page (bottom). Figure of J.L. Llácer and V. Rubio taken from Chin (2008). Reprinted with permission from AAAS (B). Cartoon representation of the S. elongatus PII-NAGK complex after removing the back NAGK dimer for clarity. The three-fold symmetry axis is vertical. Reprinted from Current Opinion in Structural Biology, 18, Llácer et al., Arginine and nitrogen storage, 673–681, 2008, with permission from Elsevier. (C) PII subunit-NAGK subunit contacts. PII, NAGK, and NAG are shown as strings, ribbons, and spheres, respectively. The contacting parts of the T-loop, B-loop, and β1–α1 connection, including some interacting side chains (in sticks), are blue, red, and green, respectively. The surfaces provided by these elements form meshworks of the same colors. The NAGK central β-sheet is green, and other β-strands and the α-helices are brownish and grayish for N- and C-domains, respectively. Some NAGK elements and PII residues are labeled. This figure and its legend reproduce with some modifications a figure and its legend of Llácer et al. (2007). The crystal structure of the complex of PII and acetylglutamate kinase reveals how PII controls the storage of nitrogen as arginine. Copyright (2007) National Academy of Sciences. (D,E), active and inactive conformations, respectively, of hexameric arginine-inhibitable NAGK. The active form is from a crystal of the enzyme from Pseudomonas aeruginosa (PDB 2BUF) while the inactive form is from the Thermotoga maritima enzyme (PDB 2BTY) (Ramón-Maiques et al., 2006). Note that the inactive form is widened relative to the active form, and that it has arginine sitting on both sides of each interdimeric junction. In the active form the nucleotide (in this case the product ADP rather than the substrate ATP) and NAG sit one in each domain of individual subunits. The NAGK observed in the PII-NAGK complex is in the active form, being stabilized in this form by its contacts with PII.