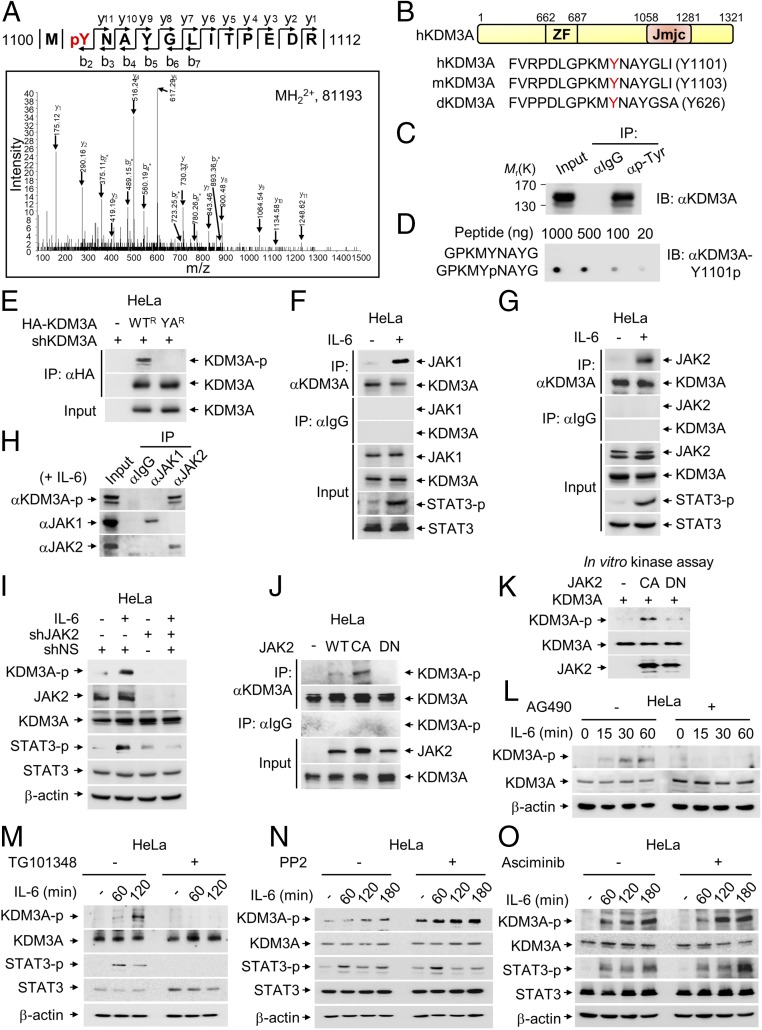
Fig. 1.
JAK2 phosphorylates KDM3A at tyrosine 1101 residue. (A) Identification by LC-MS/MS of a tyrosine phosphorylation site on KDM3A. (B) Schematic of KDM3A showing zinc finger (ZF) and Jumonji C (Jmjc) domains. Sequences around tyrosine residue (Y, marked by red) are conserved among Drosophila (d), mouse (m), and human (h). (C) Protein extracts from HeLa cells were subjected to pull-down with anti−pan-phospho-tyrosine antibody. Phosphorylation of KDM3A was assessed by immunoblot with anti-KDM3A antibody. (D) Dot blotting of unmodified or phosphorylated KDM3A peptides with anti−phospho-KDM3A (KDM3A-Y1101p) antibody. (E) Endogenous KDM3A was knocked down using shRNA, and an shRNA-resistant form of HA-KDM3A WT (WTR) or HA-KDM3A YA mutant (YAR) was reconstituted in HeLa cells. KDM3A was immunoprecipitated using an anti-HA antibody and analyzed by immunoblot with anti−phospho-KDM3A antibody. (F and G) Co-IP assay of KDM3A with (F) JAK1 or (G) JAK2 was conducted in HeLa cells with or without IL-6 treatment (50 ng/mL) for 2 h. (H) Co-IP assay of JAK1 or JAK2 with phosphorylated KDM3A was conducted in HeLa cells treated with IL-6 for 2 h. Phosphorylation level of KDM3A was assessed by immunoblot analysis using anti−phospho-KDM3A antibody. (I) Endogenous JAK2 was knocked down using shRNA, and phosphorylation level of KDM3A was assessed by immunoblot analysis using anti−phospho-KDM3A antibody in HeLa cells with or without IL-6 treatment for 2 h. (J) WT, constitutively active mutant (CA) or dominant negative mutant (DN) of JAK2 was transfected to HeLa cells. Cell lysates were immunoprecipitated with anti-KDM3A antibody followed by immunoblot analysis with anti−phospho-KDM3A antibody to detect phosphorylated KDM3A. (K) In vitro kinase assay with JAK2 CA or DN mutant using recombinant KDM3A proteins. (L–O) HeLa cells, serum-starved for 24 h, were treated with IL-6 (50 ng/mL) for indicated times. Phosphorylation levels of KDM3A were analyzed by immunoblot with anti−phospho-KDM3A antibody in the absence or presence of JAK2 inhibitors (L) AG490 (10 μM) or (M) TG101348 (2.5 μM), (N) an Src family inhibitor PP2 (10 μM), or (O) an Abl inhibitor Asciminib (250 nM).