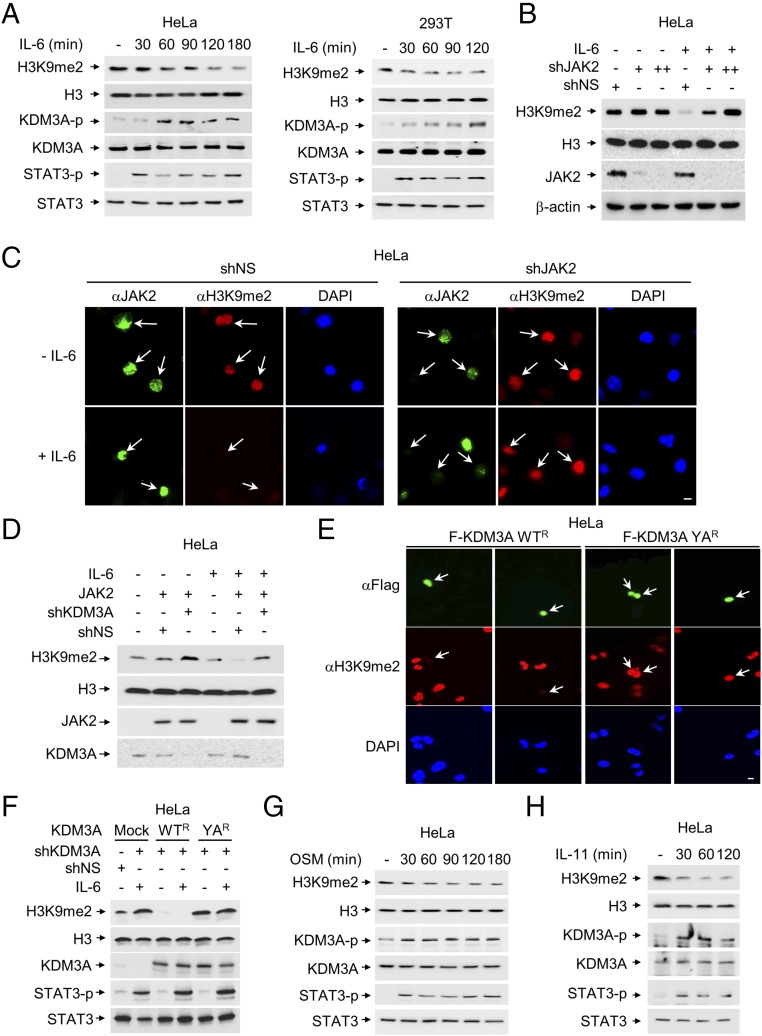
Fig. 2.
KDM3A phosphorylation by JAK2 increases its demethylase activity. (A) The level of H3K9me2 was analyzed in HeLa and HEK293T cells after IL-6 treatment. (B) The level of H3K9me2 was analyzed after knockdown of JAK2 by shRNA in the absence or presence of IL-6. (C) Representative images of cells knocked down by JAK2 shRNA in the absence or presence of IL-6. The cells were stained with anti-H3K9me2 or anti-JAK2 antibodies. Nuclei were counterstained with DAPI. Arrows indicate cells expressing JAK2 and the level of H3K9me2. (Scale bar, 10 μm.) (D) Demethylation of H3K9me2 was induced by JAK2, and knockdown of KDM3A abolished JAK2-dependent demethylation of H3K9me2 in the presence of IL-6. (E) HeLa cells were knocked down by KDM3A shRNA and reconstituted with shRNA-resistant form of KDM3A WT (WTR) or YA (YAR). Transfected cells were fixed and stained for anti-H3K9me2 and anti-Flag antibodies. The cells were counterstained with DAPI to visualize cell nuclei. Arrows indicate cells expressing KDM3A and the level of H3K9me2. (Scale bar, 10 μm.) (F) Demethylation of H3K9me2 induced by IL-6 is dependent on KDM3A. Protein extracts from HeLa cells were knocked down by KDM3A shRNA and reconstituted with KDM3A WTR or YAR. Transfected cells were collected to determine the H3K9me2 levels in the absence or presence of IL-6 by immunoblot analysis. (G and H) The level of H3K9me2 was analyzed after treatment of (G) OSM (20 ng/mL) or (H) IL-11 (5 ng/mL), which share a gp130-mediated signaling molecule in HeLa cells, serum-starved for 24 h.