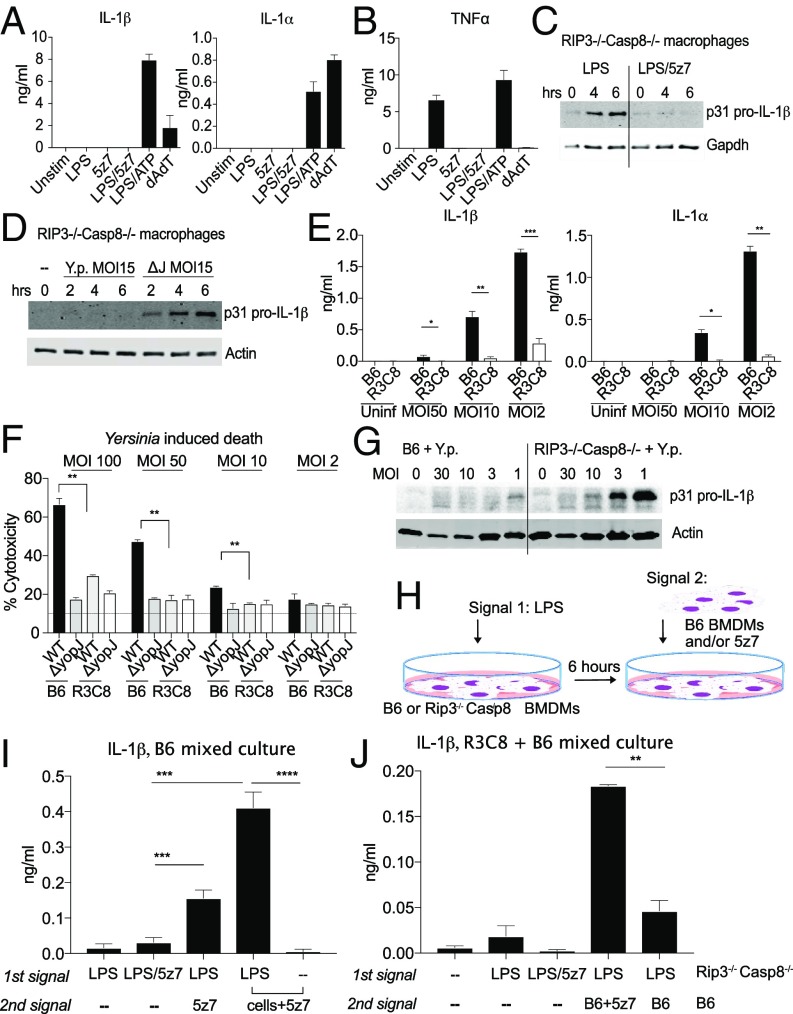
Fig. 5.
IL-1 secretion requires two distinct cell populations to generate signal 1 and signal 2. (A) Supernatant IL-1α and IL-1β measured by ELISA 6 h post stimulation by LPS/5z7 compared with canonical inflammasome stimuli. (B) TNF secretion measured by ELISA 6 h post stimulation by LPS/5z7 compared with canonical inflammasome stimuli. (C and D) Pro–IL-1β from cell lysates of Rip3−/−Casp8−/− BMDMs stimulated with agonists (C) or infected with Yersinia (MOI 15) (D) at the indicated time points. (E) Supernatant IL-1α andIL-1β measured by ELISA 16 h post Yersinia infection of B6 and Rip3−/−Casp8−/− BMDMs as a function of decreasing bacterial MOI. (F) Yersinia YopJ-induced cytotoxicity in B6 and Rip3−/−Casp8−/− BMDMs at 6 h post infection as a function of decreasing bacterial MOI. (G) YopJ-sufficient Yersinia infected at decreasing MOIs. Pro–IL-1β synthesis was measured from cell lysate of B6 and Rip3−/−Casp8−/− BMDMs (16 h). (H) Experimental schematics for I and J. (I) IL-1β ELISA from B6 BMDMs asynchronously stimulated with the indicated signals with a 1-h interval between the first and second stimulus. The last two conditions represent the addition of B6 BMDMs and 5z7 for the second signal. (J) IL-1β ELISA from mixed cultures of B6 and Rip3−/−Casp8−/− BMDMs asynchronously stimulated with the indicated signals with 1 h between the first and second stimulus. The first population of cells consists of Rip3−/−Casp8−/− BMDMs plated overnight and experiencing the first signal for 1 h before the addition of the second signal. The second signal is either absent or is present in B6 BMDMs with or without 5z7. Time-point cytotoxicity quantifications and ELISA data are shown as ± SD from three independent experiments compared using Student’s two-tailed t test: ns, nonsignificant (P > 0.05); *P < 0.05; **P < 0.01; ***P < 0.001. All Western blot data are representative of three or more experiments.