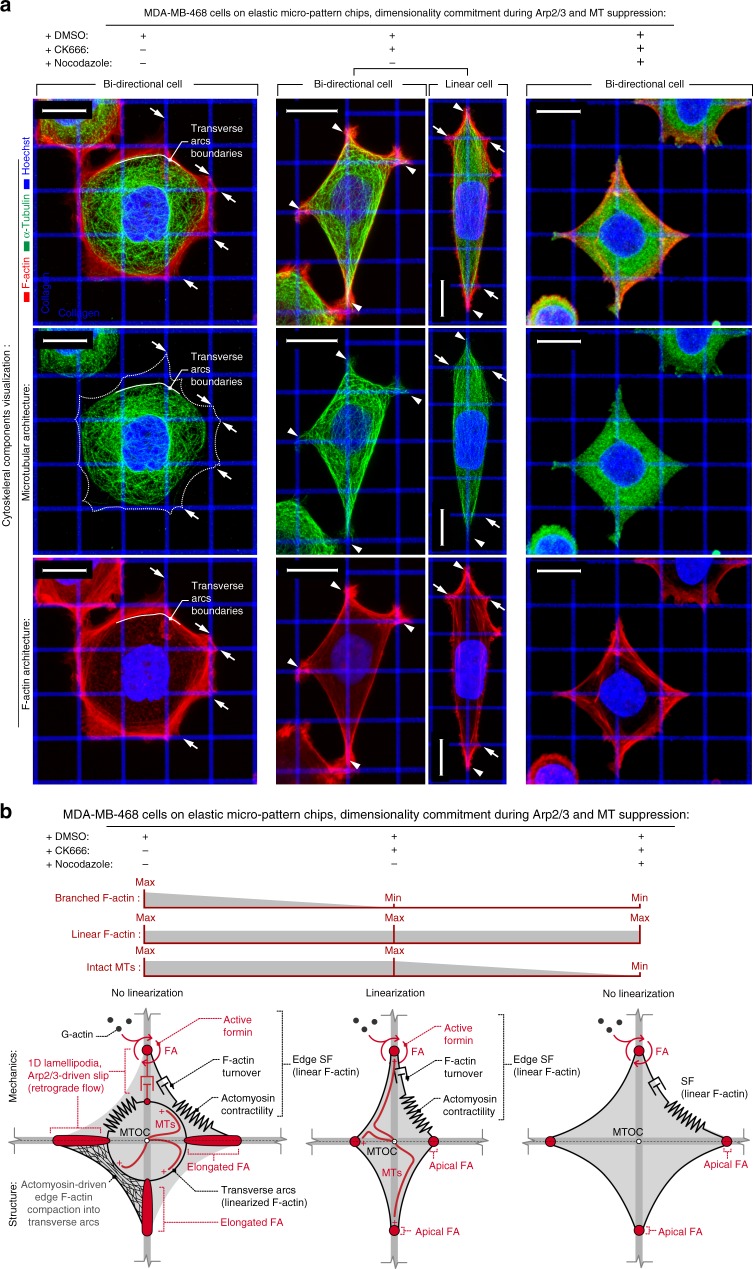
Fig. 6.
Arp2/3 suppression-induced cytoskeletal and cell architecture reorganization. a 3D reconstructions of MDA-MB-468 cells in control (+DMSO), under Arp2/3 inhibition (+CK666), and Arp2/3 inhibition without intact microtubules (+nocodazole+CK666). Control cells develop peripheral stress-fibers and also transverse arcs (see also Figs. 1c and 3a) that spatially coincide with boundaries of microtubular network, preventing microtubule localization at the cell spreading apices (white arrows), whereas Arp2/3 inhibition shifts the balance toward peripheral stress-fibers and formation of concentrated FAs at the cell periphery at points of the cell–matrix interface (see also Fig. 4f and Supplementary Figure 6). Furthermore, in Arp2/3-abrogated cells, the microtubule network conforms to the boundaries to the peripheral stress-fibers and cell apices (white arrowheads). In contrast, no intact microtubule networks are observed after nocodazole treatment. b Schematic representation of actomyosin and microtubular cytoskeleton components in control cells and in cells under Arp2/3 (±intact MTs) inhibition conditions. Note actomyosin contractility along the peripheral stress-fibers and its compaction along the transverse arcs where the transverse arcs boundaries prevent microtubule localization to the cell adhesion boundaries in control cells. Arp2/3 inhibition causes suppression of transverse arcs, allowing the microtubule network to connect to the cell adhesion boundaries, resulting in more isolated peripheral stress-fibers and diminished 1D lamellipodia slip. Removal of intact MT network rescues cells from CK666-mediated uniaxial linearization. Scale bars—15 µm