Table 1. Screening of conditions for benzylic Csp3–H arylation of N,N-dimethylbenzylamine a .
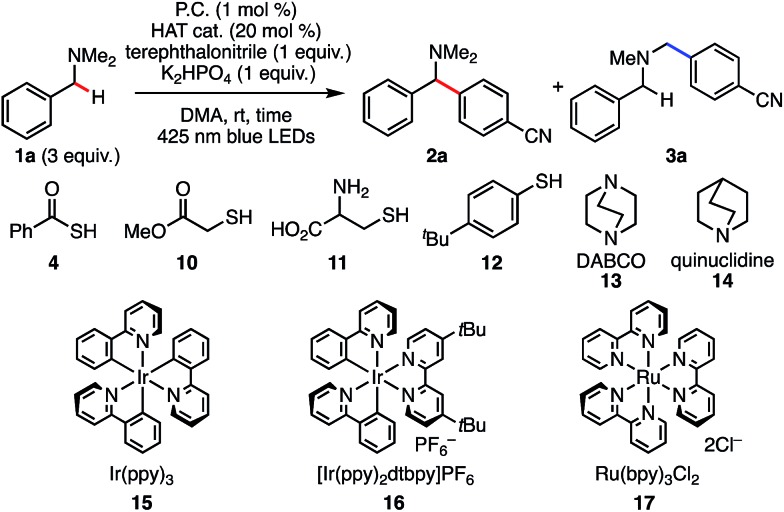
| |||||
| Entry | HAT cat. | P.C. | Time (h) | Yield b (%) | 2a : 3a b |
| 1 c | — | 15 | 12 | 95 | 1 : 8.5 |
| 2 | 4 | 15 | 12 | 91 | >20 : 1 |
| 3 | 4 | 15 | 1 | 93 | >20 : 1 |
| 4 | 10 | 15 | 1 | 23 | >20 : 1 |
| 5 | 11 | 15 | 1 | 43 | >20 : 1 |
| 6 | 12 | 15 | 1 | Trace | — |
| 7 | 13 | 15 | 1 | Trace | — |
| 8 | 14 | 15 | 1 | 12 | 1 : 2 |
| 9 | 4 | 16 | 1 | 42 | >20 : 1 |
| 10 | 4 | 17 | 1 | No reaction | |
| 11 d , e | 4 | 15 | 2 | 90 (87) f | >20 : 1 |
| 12 d , g | 4 | 15 | 2 | 72 | >20 : 1 |
aThe reactions were run on 0.2 mmol scale.
bYield and regioisomeric ratio were determined by 1H NMR analysis using 1,1,2,2-tetrachloroethane as an internal standard.
cData from Scheme 3.
d1 mol% of PhC(O)SH and 0.5 mol% of Ir(ppy)3 were used on a 1 mmol scale.
e2 equiv. of N,N-dimethylbenzylamine was used.
fIsolated yield.
g1 equiv. of N,N-dimethylbenzylamine was used. P.C.: photocatalyst.
